Abstract
Background
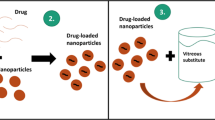
Previously, we have confirmed that the foldable capsular vitreous body (FCVB) can serve as a drug delivery system (DDS) as well as a vitreous substitute. Here, we evaluated the characteristics of the release of 5-fluorouracil (5-Fu) from FCVB in vitro and in vivo.
Methods
For the in-vitro study, various concentrations of 5-FU (50–200 μg/ml) were injected into FCVB capsules and immersed in cups of modified Franz diffusion cells, and liquid was aspirated at specific time intervals. In the in-vivo study, FCVB was folded and implanted into the vitreous cavity in the right eyes of five rabbits, and then 1.0 ml 5-Fu (200 μg/ml) was injected into the capsule. Another five rabbits that were used as the controls received intravitreal injections Aqueous humor was aspirated postoperatively at specific time intervals up to 56 days. The 5-Fu contents in vitro were detected by UV spectrophotometry and ultra performance liquid chromatography (UPLC), and the in-vivo 5-FU levels in the aqueous humour were detected by UPLC. The stock solution in the FCVB before-release study and the FCVB residue were collected for UPLC analysis.
Results
UV spectrophotometry revealed that 5-FU was released from FCVB in vitro in a time-dependent manner from 20–360 min in vitro. UPLC analysis revealed that 5-FU was released sustainably from FCVB. The 5-FU concentration in the aqueous humour was detected until postoperative day 56 (D56), with sustained release from postoperative days 3–56. However, the 5-FU concentration in the control samples was detected until only D7, and could not be detected on D14. Finally, 48.8% of the 5-FU was released on D56 in the in-vivo experiment.
Conclusions
FCVB can release 5-Fu sustainably and mechanically, indicating that FCVB can be used as a common vehicle for the sustainable release of different drugs. FCVB is a potentially valuable pharmaceutical adjunct to conventional vitreous surgery for managing or preventing proliferative vitreoretinopathy.






Similar content being viewed by others
References
Sunalp MA, Wiedemann P, Sorgente N, Ryan SJ (1985) Effect of adriamycin on experimental proliferative vitreoretinopathy in the rabbit. Exp Eye Res 41(1):105–115
Cowley M, Conway BP, Campochiaro PA, Kaiser D, Gaskin H (1989) Clinical risk factors for proliferative vitreoretinopathy. Arch Ophthalmol 107(8):1147–1151
Kirchhof B (2004) Strategies to influence PVR development. Graefes Arch Clin Exp Ophthalmol 242(8):699–703
Azen SP, Scott IU, Flynn HW Jr, Lai MY, Topping TM, Benati L, Trask DK, Rogus LA (1998) Silicone oil in the repair of complex retinal detachments. A prospective observational multi center study. Ophthalmology 105:1587–1597
Bhisitkul RB, Gonzalez VH (2005) “Heavy oil” for intraocular tamponade in retinal detachment surgery. Br J Ophthalmol 89:649–650
Leone G, Consumi M, Aggravi M, Donati A, Lamponi S, Magnani A (2010) PVA/STMP based hydrogels as potential substitutes of human vitreous. J Mater Sci Mater 21:2491–2500
Hong Y, Chirila TV, Vijayasekaran S, Shen W, Lou X, Dalton PD (1998) Biodegradation in vitro and retention in the rabbit eye of cross-linked poly (1-vinyl-2-pyrrolidinone) hydrogel as a vitreous substitute. J Biomed Mater Res 39:650–659
Wiedemann P, Heimann K (1986) Proliferative vitreoretinopathy: Pathogenesis and possibilities for treatment with cytostatic drugs. Klin Monatsbl Augenheilkd 188:559–564
Machemer R, Sugita G, Tano Y (1979) Treatment of intraocular proliferations with intravitreal steroids. Trans Am Ophthalmol Soc 77:171–180
Giordano GG, Refojo MF, Arroyo MH (1993) Sustained delivery of retinoic acid from microspheres of biodegradable polymer in PVR. Invest Ophthalmol Vis Sci 34:2743–2751
Sonoda H, Enaida H, Ueno A, Nakamura T, Kawano Y-I, Kubota T, Sakamoto T, Ishibash T (2003) Pars plana vitrectomy assisted by triamcinolone acetonide for refractory uveitis: a case series study. Br J Ophthalmol 87(8):1010–1014
Wickham L, Bunce C, Wong D, McGurn D, Charteris DG (2007) Randomized controlled trial of combined 5-Fluorouracil and low-molecular-weight heparin in the management of unselected rhegmatogenous retinal detachments undergoing primary vitrectomy. Ophthalmology 114(4):698–704
Yasukawa T, Ogura Y, Tabata Y, Kimura H, Wiedemann P, Honda Y (2004) Drug delivery systems for vitreoretinal diseases. Prog Retin Eye Res 23(3):253–281
Colthurst MJ, Williams RL, Hiscott PS, Grierson I (2000) Biomaterials used in the posterior segment of the eye. Biomaterials 21(7):649–665
Liu Y, Ke Q, Chen J, Wang Z, Xie Z, Jiang Z, Ge J, Gao Q (2010) Sustained mechanical release of dexamethasone sodium phosphate from a foldable capsular vitreous body. Invest Ophthalmol Vis Sci 51(3):1636–1642
Lin X, Ge J, Gao Q, Wang Z, Long C, He L, Liu Y, Jiang Z (2011) Evaluation of the flexibility, efficacy, and safety of a foldable capsular vitreous body in the treatment of severe retinal detachment. Invest Ophthalmol Vis Sci 52(1):374–381
Abraham LM, Selva D, Casson R, Leibovitch I (2007) The clinical applications of fluorouracil in ophthalmic practice. Drugs 67(2):237–255
Blumenkranz M, Hernandez E, Ophir A, Norton EW (1984) 5-fluorouracil: new applications in complicated retinal detachment for an established antimetabolite. Ophthalmology 91(2):122–3018
Gao Q (2008) A novel method andomould for foldable capsular vitreous body. China patent No.200810199177
Chen J, Gao Q, Liu Y, Ge J, Cao X, Luo Y, Huang D, Zhou G, Lin S, Lin J, To CH, Siu AW (2011) Evaluation of morphology and functions of a foldable capsular vitreous body in the rabbit eye. J Biomed Mater Res 97(2):396–404
Jarus G, Blumenkranz M, Hernandez E, Sossi N (1985) Clearance of intravitreal fluorouracil. Normal and aphakic vitrectomized eyes. Ophthalmology 92(1):91–96
Stern WH, Guerin CJ, Erickson PA, Lewis GP, Anderson DH, Fisher SK (1983) Ocular toxicity of fluorouracil after vitrectomy. Am J Ophthalmol 96:43–51
Stern WH, Lewis GP, Erickson PA, Guerin CJ, Anderson DH, Fisher SK, O’Donnell JJ (1983) Fluorouracil therapy for proliferative vitreoretinopathy after vitrectomy. Am J Ophthalmol 96:33–42
Rubsamen PE, Davis PA, Hernandez E, O'Grady GE, Cousins SW (1994) Prevention of experimental proliferative vitreoretinopathy with a biodegradable intravitreal implant for the sustained release of fluorouracil. Arch Ophthalmol 112:407–413
Moritera T, Ogura Y, Honda Y, Wada R, Hyon SH, Ikada Y (1991) Microspheres of biodegradable polymers as a drug-delivery system in the vitreous. Invest Ophthalmol Vis Sci 32:1785–1790
Peyman GA, Conway M, Khoobehi B, Soike K (1992) Clearance of microsphere-entrapped 5-fluorouracil and cytosine arabinoside from the vitreous of primates. Int Ophthalmol 16:109–111
Berger AS, Cheng CK, Pearson PA, Ashton P, Crooks PA, Cynkowski T, Cynkowska G, Jaffe GJ (1996) Intravitreal sustained release corticosteroid-5-fluoruracil conjugate in the treatment of experimental proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 37(11):2318–2325
Cardillo JA, Farah ME, Mitre J, Morales PH, Costa RA, Melo LAS (2004) An intravitreal biodegradable sustained release naproxen and 5-fluorouracil system for the treatment of experimental post-traumatic proliferative vitreoretinopathy. Br J Ophthalmol 88(9):1201–1205
Joondcph BC, Khoobehi B, Peyman GA, Yue BY (1998) Liposome-encapsulatcd 5-fluorouracil in the treatment of proliferative vitreoretinopathy. Ophthalmic Surg 19:252–256
Obmann A, Purevsuren S, Zehl M, Kletter C, Reznicek G, Narantuya S, Glasl S (2011) HPLC determination of flavonoid glycosides in Mongolian Dianthus versicolor Fisch. (Caryophyllaceae) compared with quantification by UV spectrophotometry. Phytochem Anal Sep 7 [Epub ahead of print]
Liu Y, Jiang Z, Gao Q, Ge J, Chen J, Cao X, Shen Q, Ma P (2010) Technical standards of foldable capsular vitreous body regarding mechanical, optical and biocompatible properties. Artif Organs 34:836–845
Gao Q, Chen X, Ge J, Liu Y, Jiang Z, Lin Z, Liu Y (2009) Refractive shifts in four selected artificial vitreous substitutes based on Gullstrand-Emsley and Liou-Brennan schematic eyes. Invest Ophthalmol Vis Sci 50:3529–3534
Verstraeten TC, Buzney SM, Macdonald SG, Neufeld AH (1990) Retinal pigment epithelium wound closure in vitro. Pharmacologic inhibition. Invest Ophthalmol Vis Sci 31(3):481–488
Acknowledgements
This study was supported by the Project of Wenzhou Municipal Science and Technology Bureau in Zhejiang province (H20080029), Natural National Science Foundation of China (NSFC----30973258) and the National Basic Research Program of China (“973”program, Number 2007CB512200).
Conflict of interest
No conflicting relationship exists for any author.
Presentation at a conference
Rabbit, ARVO.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript has not been published or submitted for publication elsewhere. We certify that all applicable institutional and governmental regulations concerning the ethical use of human subjects were followed during this research. The authors have full control of the primary data, and they agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review their data if requested. All authors equally contributed to this work.
Rights and permissions
About this article
Cite this article
Zheng, H., Wang, Z., Wang, P. et al. Evaluation of 5-fluorouracil released from a foldable capsular vitreous body in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol 250, 751–759 (2012). https://doi.org/10.1007/s00417-011-1862-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1862-y