Abstract
Purpose
To evaluate the effects of verapamil isomers on in vitro proliferation of bovine choroidal endothelial cells (CECs).
Materials and methods
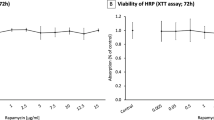
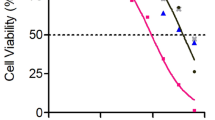
CECs were isolated from bovine eyes and cultured in endothelial growth medium (EGM). For the proliferation assays, CECs were exposed to verapamil isomers (0.1–100 μM) in EGM with 2% fetal bovine serum or basic fibroblast growth factor (bFGF) (10 ng/ml). After 72 h of incubation with the desired drug, the cellular proliferation was determined by an MTT assay and a BrdU assay. In addition, the drug toxicity on CECs stimulated with EGM was evaluated by cell counting with trypan blue.
Results
All verapamil isomers inhibited the bFGF- or medium-stimulated growth significantly in a concentration range of 10–40 μM without toxicity. No significant differences were seen between the inhibitory effects of the various isomers. Cell toxicity was detected at a concentration of 100 μM verapamil isomers on EGM-stimulated CECs.
Conclusion
The results demonstrate the efficacy of all verapamil isomers in inhibiting CEC proliferation involved in the process of choroidal neovascularization. d-(+)-Verapamil may be recommended for further in vivo evaluation in an animal model of exudative AMD; it has fewer systemic and local side effects because calcium channels are not blocked.



Similar content being viewed by others
References
Alessandro R, Spoonster J, Wersto RP, Kohn EC (1996) Signal transduction as a therapeutic target. Curr Top Microbiol Immunol 213(Pt 3):167–188
Amin R, Puklin JE, Frank RN (1994) Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Investig Ophthalmol Vis Sci 35:3178–3188
Batra S, Popper LD, Hartley-Asp B (1991) Effect of calcium and calcium antagonists on 45Ca influx and cellular growth of human prostatic tumor cells. Prostate 19:299–311
Blaheta RA, Hailer NP, Brude N, Wittig B, Leckel K, Oppermann E, Bachmann M, Harder S, Cinatl J, Scholz M, Bereiter-Hahn J, Weber S, Encke A, Markus BH (2000) In vitro analysis of verapamil-induced immunosuppression: potent inhibition of T cell motility and lymphocytic transmigration through allogeneic endothelial cells. Transplantation 69:588–597
Cattaneo MG, Gullo M, Vicentini LM (1993) Ca2+ and Ca2+ channel antagonists in the control of human small cell lung carcinoma cell proliferation. Eur J Pharmacol 247:325–331
Catterall WA, Seagar MJ, Takahashi M (1988) Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle. J Biol Chem 263:3535–3538
Eastman A, Rigas JR (1999) Modulation of apoptosis signaling pathways and cell cycle regulation. Semin Oncol 26:7–16; discussion 41–12
Echizen H, Brecht T, Niedergesass S, Vogelgesang B, Eichelbaum M (1985) The effect of dextro-, levo-, and racemic verapamil on atrioventricular conduction in humans. Am Heart J 109:210–217
Faude F, Enzmann V, Poschmann E, Hoffmann S, Wiedemann P (2000) R-(+)-verapamil, S-(−)-verapamil, and racemic verapamil inhibit human retinal pigment epithelial cell contraction. Graefes Arch Clin Exp Ophthalmol 238:537–541
Fine RL, Koizumi S, Curt GA, Chabner BA (1987) Effect of calcium channel blockers on human CFU-GM with cytotoxic drugs. J Clin Oncol 5:489–495
Frank RN (1997) Growth factors in age-related macular degeneration: pathogenic and therapeutic implications. Ophthalmic Res 29:341–353
Freshny R (1992) Animal cell culture: a practical approach. IRL Press, Oxford, England, UK
Frishman W, Kirsten E, Klein M, Pine M, Johnson SM, Hillis LD, Packer M, Kates R (1982) Clinical relevance of verapamil plasma levels in stable angina pectoris. Am J Cardiol 50:1180–1184
Giugliano G, Pasquali D, Notaro A, Brongo S, Nicoletti G, D’Andrea F, Bellastella A, Sinisi AA (2003) Verapamil inhibits interleukin-6 and vascular endothelial growth factor production in primary cultures of keloid fibroblasts. Br J Plast Surg 56:804–809
Glossmann H, Striessnig J (1988) Calcium channels. Vitam Horm 44:155–328
Grossniklaus HE, Ling JX, Wallace TM, Dithmar S, Lawson DH, Cohen C, Elner VM, Elner SG, Sternberg P Jr (2002) Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis 8:119–126
Hailer NP, Blaheta RA, Harder S, Scholz M, Encke A, Markus BH (1994) In vitro studies on immunosuppression with verapamil. Zentralbl Chir 119:347–354
Hailer NP, Blaheta RA, Harder S, Scholz M, Encke A, Markus BH (1994) Modulation of adhesion molecule expression on endothelial cells by verapamil and other Ca++ channel blockers. Immunobiology 191:38–51
Harris MS, Sakamoto T, Kimura H, He S, Spee C, Gopalakrishna R, Gundimeda U, Yoo JS, Hinton DR, Ryan SJ (1996) Hypericin inhibits cell growth and induces apoptosis in retinal pigment epithelial cells: possible involvement of protein kinase C. Curr Eye Res 15:255–262
Hockwin O, Dragomirescu V, Laser H, Ohrloff C, Kozamanoglu K, Kremer F (1984) Evaluation of the ocular safety of verapamil. Scheimpflug photography with densitometric image analysis of lens transparency in patients with hypertrophic cardiomyopathy subjected to long-term therapy with high doses of verapamil. Ophthalmic Res 16:264–275
Hoffman S, Gopalakrishna R, Gundimeda U, Murata T, Spee C, Ryan SJ, Hinton DR (1998) Verapamil inhibits proliferation, migration and protein kinase C activity in human retinal pigment epithelial cells. Exp Eye Res 67:45–52
Hoffmann S, Spee C, Murata T, Cui JZ, Ryan SJ, Hinton DR (1998) Rapid isolation of choriocapillary endothelial cells by Lycopersicon esculentum-coated Dynabeads. Graefes Arch Clin Exp Ophthalmol 236:779–784
Husain D, Miller JW, Michaud N, Connolly E, Flotte TJ, Gragoudas ES (1996) Intravenous infusion of liposomal benzoporphyrin derivative for photodynamic therapy of experimental choroidal neovascularization. Arch Ophthalmol 114:978–985
Kaumann AJ, Serur JR (1975) Optical isomers of verapamil on canine heart. Prevention of ventricular fibrillation induced by coronary artery occlusion, impaired atrioventricular conductance and negative inotropic and chronotropic effects. Naunyn Schmiedebergs Arch Pharmacol 291:347–358
Killingsworth MC, Sarks JP, Sarks SH (1990) Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye 4(Pt 4):613–621
Klein BE, Klein R, Linton KL (1992) Prevalence of age-related lens opacities in a population. The Beaver Dam Eye Study. Ophthalmology 99:546–552
Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT (1997) Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 81:154–162
Kountakis SE, Chang CY, Minotti AM, Cabral FR (2000) Effect of verapamil on cholesteatoma migration in vitro. Otolaryngol Head Neck Surg 122:91–95
Lu WL, Dong YL (2003) Endothelial cell proliferation stimulated by basic fibroblast growth factor. Zhongguo Xiufu Chongjian Waike Zazhi 17:386–387
Meirelles Pereira LM, Mandarim-de-Lacerda CA (2000) Effect of antihypertensive drugs on the myocardial microvessels in rats with nitric oxide blockade. Pathol Res Pract 196:305–311
Nishimura T, Zhu ZR, Ryan SJ (1990) Effects of sodium iodate on experimental subretinal neovascularization in the primate. Ophthalmologica 200:28–38
Penfold PL, Provis JM, Billson FA (1987) Age-related macular degeneration: ultrastructural studies of the relationship of leucocytes to angiogenesis. Graefes Arch Clin Exp Ophthalmol 225:70–76
Rasmussen H, Barrett PQ (1984) Calcium messenger system: an integrated view. Physiol Rev 64:938–984
Richter D, Hatvani I, Toth A (1993) Growth inhibition of intraocular proliferative explants under in vitro conditions by verapamil. Klin Monatsbl Augenheilkd 203:206–211
Spedding M, Paoletti R (1992) Classification of calcium channels and the sites of action of drugs modifying channel function. Pharmacol Rev 44:363–376
Steinleitner A, Lambert H, Kazensky C, Sanchez I, Sueldo C (1990) Reduction of primary postoperative adhesion formation under calcium channel blockade in the rabbit. J Surg Res 48:42–45
Sunderkotter C, Goebeler M, Schulze-Osthoff K, Bhardwaj R, Sorg C (1991) Macrophage-derived angiogenesis factors. Pharmacol Ther 51:195–216
Takahashi K, Itagaki T, Yamagishi K, Ohkuma H, Uyama M (1990) The role of retinal pigment epithelium in the early stage of development of subretinal neovascularization. Nippon Ganka Gakkai Zasshi 94:3–17
Thomas MA, Grand MG, Williams DF, Lee CM, Pesin SR, Lowe MA (1992) Surgical management of subfoveal choroidal neovascularization. Ophthalmology 99:952–968; discussion 975–956
Uyama M (1991) Choroidal neovascularization: experimental and clinical study. Nippon Ganka Gakkai Zasshi 95:1145–1180
Wagner M, Benson MT, Rennie IG, MacNeil S (1995) Effects of pharmacological modulation of intracellular signalling systems on retinal pigment epithelial cell attachment to extracellular matrix proteins. Curr Eye Res 14:373–384
Walz G, Zanker B, Barth C, Wieder KJ, Clark SC, Strom TB (1990) Transcriptional modulation of human IL-6 gene expression by verapamil. J Immunol 144:4242–4248
Yohem KH, Clothier JL, Montague SL, Geary RJ, Winters AL III, Hendrix MJ, Welch DR (1991) Inhibition of tumor cell invasion by verapamil. Pigment Cell Res 4:225–233
Zeitler H, Ko Y, Glodny B, Totzke G, Appenheimer M, Sachinidis A, Vetter H (1997) Cell-cycle arrest in G0/G1 phase of growth factor-induced endothelial cell proliferation by various calcium channel blockers. Cancer Detect Prev 21:332–339
Zernig G (1990) Widening potential for Ca2+ antagonists: non-L-type Ca2+ channel interaction. Trends Pharmacol Sci 11:38–44
Zhou X (1992) Tetrandrine on monocyte migration induced by endothelial cell-derived chemotactic factor. Zhonghua Yixue Zazhi 72:424–426, 447
Acknowledgements
This work was supported in part by a grant from the German Research Association (DFG) (WI 880\9-1) and a junior grant from the University of Leipzig (no. 78311106).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoffmann, S., Balthasar, S., Friedrichs, U. et al. Inhibitory effects of verapamil isomers on the proliferation of choroidal endothelial cells. Graefe's Arch Clin Exp Ophthalmo 244, 376–381 (2006). https://doi.org/10.1007/s00417-004-1104-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-1104-7