Abstract
Motor–cognitive training in Parkinson’s disease (PD) can positively affect gait and balance, but whether motor–cognitive (dual-task) performance improves is unknown. This meta-analysis, therefore, aimed to establish the current evidence on the effects of motor–cognitive training on dual-task performance in PD. Systematic searches were conducted in five databases and 11 studies with a total of 597 people (mean age: 68.9 years; mean PD duration: 6.8 years) were included. We found a mean difference in dual-task gait speed (0.12 m/s (95% CI 0.08, 0.17)), dual-task cadence (2.91 steps/min (95% CI 0.08, 5.73)), dual-task stride length (10.12 cm (95% CI 4.86, 15.38)) and dual-task cost on gait speed (− 8.75% (95% CI − 14.57, − 2.92)) in favor of motor–cognitive training compared to controls. The GRADE analysis revealed that the findings were based on high certainty evidence. Thus, we can for the first time systematically show that people with PD can improve their dual-task ability through motor–cognitive training.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Everyday life requires us to perform tasks simultaneously and pay attention while doing so. This act of dual-tasking is by definition conducted when two tasks with distinct goals are performed simultaneously [1]. People with neurological disorders typically experience greater difficulties while dual-tasking compared to healthy controls [2, 3]. In Parkinson’s disease (PD) specifically, gait impairments are well documented and show that during dual-task walking conditions, gait speed [4,5,6,7,8,9,10,11,12,13,14] and step length [4,5,6,7,8,9,10,11,12,13,14] decrease, while gait variability [4, 8, 9, 12,13,14], and the number of freezing episodes increase [15]. Interview studies with people with PD elucidate the need to concentrate to maintain a basic walking rhythm even at early stages of the disease, [16] and to use self-talk to anticipate and plan for the next step ahead [17].
Although not yet fully understood, it is believed that executive dysfunction, together with a gradual loss of automaticity in PD may partly explain the impaired ability to dual task [18]. According to the model proposed by Fitts and Posner (1967), motor learning occurs through a three-stage process [19]. At first, we familiarize ourselves with the task through conscious performance and information processing (cognitive stage). In the second, associative stage, we start carrying out the task, adjust it and finetune its performance. Finally, in the autonomous stage, the task can be performed with minimal cognitive and attentional demand [19]. As a movement becomes automatic, imaging studies on the healthy brain have shown that brain activity in the dorsolateral prefrontal cortex and the anterior cingulate cortex decreases, whereas connectivity increases between the putamen and different motor areas. However, in PD, due to dopamine depletion in the putamen, no such connectivity increase occurs, resulting in difficulties acquiring automaticity [18, 20].
The extent to which people with PD can improve their ability to dual task through training is unclear, despite an increase in the number of trials focusing on motor–cognitive training during the past decade. This is because previous efforts to systematically study the effects of motor–cognitive training in PD [21,22,23] have focused on effects on single-task gait and balance, and not on dual-task performance per se. In consideration of the principles of motor learning, combining motor and cognitive training (motor–cognitive training) should provide the added advantage of task specificity, when compared to the consecutive training of these tasks. However, it has also been debated whether in certain PD subgroups, such as individuals with cognitive impairment or those suffering from freezing of gait, consecutive training is a safer option [24]. The prevalence of both these symptoms is reported as 40%, even at early disease stages [25, 26], and increases in line with disease progression [26, 27].
In older adults at various stages of cognitive impairment, motor–cognitive training has shown beneficial effects for both physical [28, 29] and cognitive function, as well as in reducing dual-task cost on gait speed (i.e., the proportion by which gait speed is reduced compared to single-task walking) [28]. Interestingly, motor–cognitive training also appears to have a larger effect on executive function than cognitive training alone which suggests that physical exercise might act as an aggregate [30]. Nonetheless, due to the lack of systematic evidence to support whether dual-task training techniques can improve motor–cognitive function in PD, it is unknown whether these more complex interventions lead to benefits for this group when performing dual-task activities. Thus, the aim of this systematic review and meta-analysis is to establish the current evidence on the effects of motor–cognitive training on dual-task performance in people with PD.
Methods
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [31]. It was preregistered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42021278518).
Keywords, databases, and review process
Searches were conducted by information specialists in the following databases up to September 28th, 2021, CINAHL, Web of Science, MEDLINE Ovid and Cochrane Central Register of Controlled Trials (CENTRAL). Reference lists of all studies that were found to be eligible for this review were hand searched for further eligible trials. Language was restricted to English, Swedish, and German. No restrictions were set for publication date. Databases were searched with an elaborate search string (see Online Resource 1).
Studies retrieved through the electronic database searches were screened based on title and abstract independently by two review authors (HJ and IH) in Rayyan [32]. After completed screening, authors were unblinded to each-others’ decisions. Disagreements were discussed and resolved with other members (BL, EK and AKF) of the review team. After the initial screening, a full text review of the included studies was performed independently by two authors (HJ and IH), and unblinded upon completion. In case of uncertainty, further review authors (BL, EK and AKF) were consulted until consensus was reached. Final decisions for inclusion were hereafter discussed within the review team.
Eligibility criteria
Eligible study designs were randomized controlled trials (RCT) or quasi-RCT. Reviews and meta-analysis were excluded as well as letters to the editors, comments, conference posters, and further conference contributions, study protocols and trial register entries, books and book chapters were excluded. Studies were eligible for inclusion if they were conducted on human adults (≥ 18 years) of all sexes with a clinical diagnosis of idiopathic PD. Data from patients with atypical, genetic, or secondary Parkinsonism were not included. Regarding interventions, we focused on motor–cognitive training (dual-task training), i.e., training involving motor tasks (e.g., walking) and cognitive tasks (e.g., counting down) performed simultaneously. Motor–cognitive interventions were eligible regardless of approach (e.g., traditional or virtual reality/exergaming) and setting (in-clinic or home-based), but the minimum number of training sessions needed to be ≥ 2. Both passive (no exercise or other type of organized activity) and active (e.g., exercise without elements of dual-tasking, or education) control groups were eligible. Reporting of any type of measurable dual-task performance (e.g., dual-task gait speed) was required for inclusion.
Data extraction
A pre-piloted form was used to extract data from the included studies. Extracted information included: participant demographics, details of disease stage and cognitive function, details of the motor–cognitive intervention and the control intervention; details of the dual-task outcomes pre and post intervention pertaining to both motor task and cognitive task.
One review author (IH) extracted the data, and another reviewer (HJ) double-checked it. Ambiguity was resolved through discussion where necessary. Missing data was requested from study authors. If data were still not obtained after two reminders to study authors, the data were considered as missing. Data were requested from six reports [33,34,35,36,37,38], whereof data from four reports were retrieved [33, 36,37,38].
Data analyses
Three studies had more than one report included, and for this reason only the main reports [36, 38,39,, 40] were referenced for sample characteristics, and more than one report for each study never included in the same meta-analysis. One study [36] was a cross-over RCT, and therefore only midpoint data was used in the meta-analyses. Descriptive data on study participants were pooled among the studies (i.e., not reports).
Review Manager (RevMan) version 5.4 was used for meta-analyses [41]. The outcomes available for meta-analyses (dual-task gait speed, dual-task cadence, dual-task stride length, dual-task stride length standard deviation (SD), dual-task stride time SD, dual-task double support, dual-task cost on gait speed, dual-task reaction time, Timed Up and Go cognitive (TUG cog)) were continuous and treatment effect measures were therefore given as mean differences (MDs) with 95% confidence intervals (CI). As we expected some heterogeneity in the trial designs, a random-effect model was used. Additional outcomes that were only reported by a single study (i.e., dual-task cost on stride length, dual-task accuracy, dual-task cost on accuracy, and dual-task unipedal stance test) were also meta-analyzed and can be found in Online Resource 2, Fig. 1a–d. Two types of sensitivity analyses were performed; one comparing meta-analyses using fixed-effect models, and one comparing meta-analyses with and without including studies using a passive control group [38].
Risk of bias assessment
Risk of bias was assessed on outcome level (dual-task performance) using Cochrane Risk of Bias tool 2.0 (RoB2) which considers bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, bias in selection of the reported result and overall risk of bias. Two authors (HJ and BL) assessed risk of bias using RoB2 independently and unblinded after completion, whereafter any discrepancies were resolved through discussion with a third author (AKF).
In accordance with our protocol registration, no funnel plots for publication bias were created as each of the meta-analyses performed included less than ten studies according to the Cochrane Handbook for Systematic Reviews of Interventions [42]. Publication bias was instead assessed by searching trial registries (ClinicalTrials.gov and International Clinical Trials Registry Platform (ICTRP)) to identify completed but not published trials. In cases where no publication could be retrieved from a completed trial, the principal investigator of the respective trials was contacted in order to obtain more information. Principal investigators of the following trial register entries were contacted: NCT03902990, RBR-365tkt, NCT01156714, and NCT02904837, without response.
Two authors (HJ and BL) independently assessed the certainty of the body of evidence for studies that contributed to the meta-analyses using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias). The GRADEpro GDT software was used to prepare the Summary of findings tables (GRADEpro GDT 2022) [43]. Any decisions to downgrade the certainty of studies were justified in footnotes.
Results
Study selection
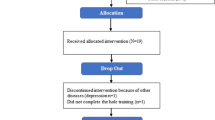
The database searches up to September 2021 yielded a total of 2252 records after duplicates were removed. A total of 2136 records were excluded based on the aforementioned eligibility criteria, leaving 116 records for full text evaluation. During full text evaluation, 99 reports were excluded, see Online Resource 3, Table 1, for detailed information on reasons for exclusion. A final of 11 studies (reported in 17 articles) were included for further qualitative and quantitative analyses. See Fig. 1 for a PRISMA flow diagram of the screening process.

Modified from Page et al. [44]
PRISMA flow diagram of the screening process.
Study characteristics
Population characteristics
All included studies were of RCT design and had been conducted in the following countries: Belgium, Israel, the Netherlands, Spain, Sweden, Taiwan, and the United States of America (USA). A total of 597 people were randomized into the studies, for which descriptive characteristics were reported in 573 (37.2% women) and 564 were analyzed post the interventions. Participants included in the studies had a pooled mean age of 68.9 years (pooled SD 7.4), a pooled mean H&Y of 2.0 (pooled SD 0.4), and a pooled mean disease duration of 6.8 years (pooled SD 4.8). Six reports [35,36,37, 40, 45, 46] from two main studies [36, 40] stated the percentage of individuals suffering from freezing of gait (FOG) in their respective samples. Five reports [35, 36, 46,47,48] described FOG symptoms using either the freezing of gait questionnaire (FOGQ) [47, 48] or the new FOGQ (NFOGQ) [35, 36, 46]. Two studies used severe motor fluctuations as an exclusion criterion [47, 48], but none of the included studies described the occurrence or severity of motor fluctuations in their respective samples. Levodopa equivalent daily dosage (LEDD) was described in 11 [33, 38,39,40, 45,46,47,48,49,50,51] of the 17 reports. Nine studies used a cutoff for global cognition as an inclusion criteria [33, 34, 38, 40, 48, 49, 51,52,53], one study [36] used ability to consent and follow testing and intervention procedures as an inclusion criteria, and one study [47] did not specify any exclusion based on cognitive ability. For information on individual reports and characteristics on included participants, see Table 1.
Interventions and comparisons
The content of the motor–cognitive interventions varied and included highly challenging dual-task balance training [38, 49], circuit training progressed with cognitive dual tasks [36], treadmill training with virtual reality (VR) [33], Wii-based motor and cognitive training [34], dual-task gait training [40, 48, 52], and balance training with VR [53]. The dose ranged between 30 and 80 min (mean 51.8), 2–4 times per week (mean 2.6) for 4–12 weeks (mean 7.7). Two studies used an added home training program to the motor–cognitive intervention to be performed for an additional 60 min per week [40, 49]. Six of the interventions were conducted in a group setting [36, 38, 49, 52], and five as individual training [33, 34, 40, 48, 53]. Two studies used a passive control group [38, 47], one study used both a passive and an active control group [53], seven studies used an active control group [33, 34, 36, 40, 49, 52], and one study used two different active control groups [48]. Active control group content included gait and cognitive training performed consecutively [40, 51], gait training [52], motor dual-task gait training [48], balance training [53], treadmill training [33], global exercises and balance training [34], speech and communication therapy [49], and education [36]. The dose of the active control group interventions ranged between 30 and 80 min (mean 48.3), 1–4 times per week (mean 2.4) for 4–10 weeks. Three studies used an added home training program in addition to the active control group training, and these increased the weekly training by 60–180 min. For detailed information on the content of each intervention, see Table 2.
Outcomes of dual-task performance
Various spatiotemporal aspects of gait during dual-task walking were the most commonly reported outcomes of dual-task performance in the included studies. Most frequent was the reporting of dual-task gait speed, followed by cadence, stride (or step) length, percent of the gait cycle spent in double support, and stride length- and stride time variability (standard deviations). Five studies reported dual-task performance of a cognitive task (e.g., reciting every other letter of the alphabet, an auditory Stroop task, and a subtraction task) that was carried out simultaneously with a motor task, and presented in terms of accuracy rate, dual-task cost on accuracy rate, error rate and/or reaction time [36, 38, 40, 49, 53]. Other assessments evaluating dual-task performance included the Timed Up and Go cognitive (TUG cog), a dual-task test of functional mobility, and the unipedal stance test performed with a simultaneous cognitive task.
Results of syntheses
Dual-task gait speed
Eight studies were included in the meta-analysis for the outcome dual-task gait speed [33, 36, 38, 40, 48, 49, 51, 52], see Fig. 2a. In terms of overall risk of bias, two studies were assessed as having high risk of bias, six studies some concerns, and one study was considered to have low risk of bias. The random-effects model showed a significant mean difference in gait speed of 0.12 m/s (95% CI 0.08, 0.17) in favor of the motor–cognitive training in contrast to passive and active control groups.
Forest plot. a Motor–cognitive training vs control. Outcome: dual-task gait speed. b Motor–cognitive training vs control. Outcome: dual-task cadence. c Motor–cognitive training vs control. Outcome: dual-task stride length. d Motor–cognitive training vs control. Outcome: dual-task stride length SD. e Motor–cognitive training vs control. Outcome: dual-task stride time SD. f Motor–cognitive training vs control. Outcome: dual-task double support. g Motor–cognitive training vs control. Outcome: dual-task cost on gait speed. h Motor–cognitive training vs control. Outcome: dual-task reaction time. i Motor–cognitive training vs control. Outcome: Timed Up and Go cognitive
Dual-task cadence
Five studies were included in the meta-analysis for the outcome dual-task cadence [38, 45, 48, 51, 52], see Fig. 2b. Two studies had high overall risk of bias, two studies were assessed as some concerns, and one study as low. The random-effect model showed a significant mean difference cadence of 2.91 steps/min (95% CI 0.08, 5.73) in favor of the motor–cognitive training in contrast to passive and active control groups.
Dual-task stride length
Six studies were included in the meta-analysis for the outcome stride length [33, 36, 38, 45, 48, 52], see Fig. 2c. Two studies had high overall risk of bias, three studies were assessed as some concerns, and one study as low. The random-effect model showed a significant mean difference in stride length of 10.12 cm (95% CI 4.86, 15.38) in favor of the motor–cognitive training in contrast to passive and active control groups.
Dual-task gait variability
Two studies reported synthesizable data on gait variability [45, 50]. One study had low overall risk of bias, and one had some concerns. Neither of the two random-effect models showed significant results regarding stride length SD (p = 0.42), Fig. 2d, or stride time SD (p = 0.26), Fig. 2e.
Dual-task double support
Three studies were included in the meta-analysis for the outcome double support [38, 45, 48], see Fig. 2f. One study had low overall risk of bias, one had some concerns, and one had high. The random-effects model was not significant (p = 0.13) regarding any mean difference in double support between motor–cognitive training and control.
Dual-task cost on gait speed
Two studies were included in the meta-analysis for the outcome dual-task cost on gait speed [36, 48], see Fig. 2g. Both studies had some concerns in overall risk of bias. The random-effects model showed a significant mean difference in dual-task cost on gait speed of − 8.75% (95% CI-14.57, − 2.92) in favor of the motor–cognitive training in contrast to active control groups.
Dual-task reaction time
Two studies were included in the meta-analysis for the outcome reaction times of the cognitive task during dual-tasking [40, 53], see Fig. 2h. The random-effects model was not significant (p = 0.47) regarding any mean difference in reaction time between motor–cognitive training and control.
Timed Up and Go cognitive
Three studies were included in the meta-analysis for the outcome TUG cog [38, 47, 49], see Fig. 2i. Regarding overall risk of bias, two of the studies were assessed as having high risk of bias, and one study as some concerns. The random-effects model was not significant (p = 0.17) regarding any mean difference in performance between motor–cognitive training and control.
Sensitivity analysis
The results of the sensitivity analyses showed a significant training effect on TUG cog using a fixed-effects model, but not using a random-effects model (the fixed-effects model showed a significant mean difference in TUG cog of − 2.16 s (95% CI − 4.05, − 0.27) in favor of the motor–cognitive training). Regarding time spent in double support (%), the fixed-effects model showed significant mean difference (− 1.19% (95% CI − 2.44, 0.05)), but the random-effects model did not. The sensitivity analyses further showed a significant mean difference in dual-task cadence with passive controls included [38], but not significant without (p = 0.07). No other differences between random and fixed-effects models, or with/without passive control groups were found. See Online Resource 4, Tables 2 and 3, for a detailed outline of the sensitivity analyses.
Risk of bias
We used the RoB2 tool to assess risk of bias for each of the included reports. A summary of the assessments is provided in Fig. 3. A majority of the reports (10/17) were considered to have some concerns in terms of overall risk of bias, and four reports were assessed as having a high overall risk of bias. In all but three of the reports assessed as either high risk of bias or some concerns, the overall risk of bias was driven by the domain pertaining to selection of the reported results. A lack of preregistered or published analysis plan was the primary reason for raised concern regarding selection of the reported results.
Certainty of evidence
Compared with no training or training without elements of dual-tasking, high certainty evidence suggests that motor–cognitive training increases dual-task gait speed, dual-task cadence, and dual-task step length, and decreases dual-task cost on gait speed (Table 3).
Discussion
The aim of this systematic review was to establish the current evidence on the effects of motor–cognitive training on dual-task performance in people with PD. To the best of our knowledge, this is the first systematic review and meta-analysis providing evidence that people with PD can improve their dual-task abilities through motor–cognitive training. The results show that the spatiotemporal gait parameters speed, cadence and stride length increased, while dual-task cost on gait speed decreased, in comparison to passive and/or active controls. No effects were shown on measures of gait variability or percentage of time spent in double support. Results regarding TUG cog were conflicting, with the fixed-effects model significant and the random-effects model not.
Gait speed is an important biomarker of mobility and is widely accepted as the sixth vital sign [55]. Encouragingly, but in contrast to the one previous meta-analysis investigating the effect of motor–cognitive training on dual-task gait speed [22], we did find an effect compared to controls. Although the analyses do not disclose the mean increase in gait speed or contrast the results to single-task gait speed, the results are the first to show that motor–cognitive training can impact bradykinetic dual-task gait. Speed, step length and cadence are highly interrelated and so it is unsurprising that improvements in respective gait parameter follow a similar pattern. The clinically meaningful difference in single-task gait speed in PD ranges from 0.06 m/s (small effect) to 0.22 m/s (large effect) [55], but no such cut-offs are currently available for dual-task gait speed. To what extent the cut-offs can help the interpretation of differences in dual-task gait speed is unclear. Previous research have shown that although single and dual-task gait speeds are highly related [57], the latter is also associated with, for example, executive function [57] and functional balance [58]. The analyses did not indicate any post-intervention, across group differences in gait variability. Although these two meta-analyses are based on two studies only, the results are disconcerting as gait variability is associated with both gait automaticity [59] and fall risk [60].
According to a survey of physiotherapists working with PD patients in Sweden, the most commonly used standardized measurement tool was the TUG test (used by ≥ 97%) [61]. Both the TUG [62] and the TUG with an added cognitive task (TUG cog) [63] can also help identify individuals with PD with a high or low risk of falls. The result of this study showed that the group participating in a motor–cognitive intervention took in mean 2.6 fewer seconds to complete the TUG cog compared to controls (using a fixed-effects model). This may be of clinical importance as deterioration of gait under dual-task straight walking have not shown an association to prospective falls [62]. The TUG test presumably mirrors everyday indoor movements to a larger extent than straight walking tests. Evaluating dual-task performance after motor–cognitive interventions is important, but perhaps a test of straight walking is insufficient if we also want to predict whether the training can affect fall risk in a PD population.
Participants in the studies included in this review typically had mild to moderate disease severity (mean H&Y 2.0). Although this is reflective of PD exercise trials in general, this mild-moderate sample diminishes the ability to understand how motor–cognitive training affects de novo PD or people with severe disease severity. Interestingly, responder analyses from two of the included studies indicate that those benefiting most from motor–cognitive training were people who at baseline had higher global cognition [46], lower dual-task gait speed [46, 50], and took longer time to complete the TUG test [50]. In PD, higher levels of cognitive function, and especially episodic memory and attention, is associated with a faster motor learning acquisition rate [64]. This reliance on episodic memory for motor learning in PD has been suggested as a cognitive compensatory strategy [64], and could partly explain why preserved cognition could be critical for better outcomes after motor–cognitive training. The combined interpretation of findings from these studies suggests that people with impaired motor performance but who have preserved cognition, may be the most suitable target group for motor–cognitive training.
Exploring which type and dose of motor–cognitive training that has the best effect on dual-task performance is beyond the scope of this systematic review. As the field progresses and more high-quality trials are published, it will, however, be of great importance to update and perform such analyses. The interventions described in this review varied in nature, dose, and setting which may have contributed to a clinical heterogeneity. As most included studies had not reported on the amount of time per session spent dual-tasking it is also impossible to infer what proportion of dual-tasking is sufficient. For future studies, we recommend that information regarding the mean individual training dose is included. Data from future studies should provide sufficient power to perform meta-analyses in which subgroup analyses regarding different dual-task training paradigms and intervention doses can be calculated.
There was an overall lack of reporting of performance on the cognitive task in the studies. Such information is crucial for several reasons and should always be considered when designing and/or deciding on a suitable assessment method of dual-tasking. Using standardized and reliability tested cognitive tasks such as the digit span or auditory Stroop [65] allows researchers to evaluate performance on both the motor task and the cognitive task. By doing so, the interpretation of a dual-task gait analyses can be better nuanced and reveal patterns of postural strategies and prioritization.
In the included studies, there was an overall lack of a preregistered or published analysis plan causing concerns during risk of bias assessment in the domain pertaining to selection of the reported results. With the rapid acceleration of open science over the last decade the practice of preregistration has undoubtedly increased. However, several studies in this review were published before such approaches were custom or advocated.
Whereas previous reviews on motor–cognitive training in PD have investigated the general effects on e.g., gait and balance, this is the first study focusing on actual dual-task performance. Although potential transfer effects are interesting, we believe that our novel findings of task-specific effects on dual-task performance after motor–cognitive training in PD is of even higher clinical relevance. Collating all available evidence on a certain topic does however not come without challenges. The search strategy, although striving to be broad in scope, may have failed to identify suitable motor–cognitive interventions if the authors had not described them as such, or as addressing dual-task components. We did however also perform manual searches of the reference lists as well as checking registers for ongoing trials. A total of only 11 studies were included which ultimately limited the ability to perform subgroup analyses. Our results can, therefore, not reveal for example whether people in early versus advanced disease stages, or individuals with or without freezing of gait, benefit differently from motor–cognitive training. A further limitation to this review is that the impact of cognitive state cannot be defined, due to the fact that the cognitive profile of patients is poorly or not described in most studies. Future studies should, therefore, report information on whether study participants suffered from cognitive dysfunction (i.e., subjective cognitive decline, mild cognitive impairment, or dementia) and analyze in which way cognitive state is related to motor-cognitive training response. Finally, the impact of sociodemographic factors including age, education, and sex should be considered in future research. With the rapid advancement in the field and several ongoing trials focusing on motor–cognitive interventions, future systematic reviews will have the opportunity to explore these issues.
This is the first systematic review to show that motor–cognitive interventions as compared with no training or training without elements of dual-tasking have the ability to improve various spatiotemporal aspects of dual-task gait in people with PD. As more studies become available for meta-analysis, future research should focus on discerning who benefits most from this type of intervention, as well as exploring knowledge gaps concrening optimal dose and approach.
Data availability
Data can be made available upon reasonable request.
References
McIsaac TL, Lamberg EM, Muratori LM (2015) Building a framework for a dual task taxonomy. Biomed Res Int 2015:591475
Amboni M, Barone P, Hausdorff JM (2013) Cognitive contributions to gait and falls: evidence and implications. Mov Disord 28:1520–1533
Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J (2011) Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev 35:715–728
Plotnik M, Giladi N, Hausdorff JM (2009) Bilateral coordination of gait and Parkinson’s disease: the effects of dual tasking. J Neurol Neurosurg Psychiatry 80:347–350
Plotnik M, Dagan Y, Gurevich T, Giladi N, Hausdorff JM (2011) Effects of cognitive function on gait and dual tasking abilities in patients with Parkinson’s disease suffering from motor response fluctuations. Exp Brain Res 208:169–179
Lord S, Rochester L, Hetherington V, Allcock LM, Burn D (2010) Executive dysfunction and attention contribute to gait interference in “off” state Parkinson’s disease. Gait Posture 31:169–174
Yogev-Seligmann G, Giladi N, Gruendlinger L, Hausdorff JM (2013) The contribution of postural control and bilateral coordination to the impact of dual tasking on gait. Exp Brain Res 226:81–93
Rochester L, Galna B, Lord S, Burn D (2014) The nature of dual-task interference during gait in incident Parkinson’s disease. Neuroscience 265:83–94
Stegemoller EL, Wilson JP, Hazamy A, Shelley MC, Okun MS, Altmann LJ, Hass CJ (2014) Associations between cognitive and gait performance during single- and dual-task walking in people with Parkinson disease. Phys Ther 94:757–766
Penko AL, Streicher MC, Koop MM, Dey T, Rosenfeldt AB, Bazyk AS, Alberts JL (2018) Dual-task interference disrupts Parkinson’s gait across multiple cognitive domains. Neuroscience 379:375–382
Wild LB, de Lima DB, Balardin JB, Rizzi L, Giacobbo BL, Oliveira HB, Argimon IID, Peyre-Tartaruga LA, Rieder CRM, Bromberg E (2013) Characterization of cognitive and motor performance during dual-tasking in healthy older adults and patients with Parkinson’s disease. J Neurol 260:580–589
Alcock L, Galna B, Lord S, Rochester L (2016) Characterisation of foot clearance during gait in people with early Parkinson’s disease: deficits associated with a dual task. J Biomech 49:2763–2769
Gassner H, Marxreiter F, Steib S, Kohl Z, Schlachetzki JCM, Adler W, Eskofier BM, Pfeifer K, Winkler J, Klucken J (2017) Gait and cognition in Parkinson’s disease: cognitive impairment is inadequately reflected by gait performance during dual task. Front Neurol 8:550
Johansson H, Ekman U, Rennie L, Peterson DS, Leavy B, Franzen E (2021) Dual-task effects during a motor-cognitive task in Parkinson’s disease: patterns of prioritization and the influence of cognitive status. Neurorehabil Neural Repair 35:356–366
Lord SR, Bindels H, Ketheeswaran M, Brodie MA, Lawrence AD, Close JCT, Whone AL, Ben-Shlomo Y, Henderson EJ (2020) Freezing of gait in people with parkinson’s disease: nature, occurrence, and risk factors. J Parkinsons Dis 10:631–640
Jones D, Rochester L, Birleson A, Hetherington V, Nieuwboer A, Willems AM, Van Wegen E, Kwakkel G (2008) Everyday walking with Parkinson’s disease: understanding personal challenges and strategies. Disabil Rehabil 30:1213–1221
Johansson H, Franzen E, Skavberg Roaldsen K, Hagstromer M, Leavy B (2019) Controlling the uncontrollable: perceptions of balance in people with Parkinson disease. Phys Ther 99:1501–1510
Wu T, Hallett M, Chan P (2015) Motor automaticity in Parkinson’s disease. Neurobiol Dis 82:226–234
Fitts PM, Posner MI (1967) Human performance. Brooks/Cole Pub. Co., Belmont, Calif
Wu T, Chan P, Hallett M (2010) Effective connectivity of neural networks in automatic movements in Parkinson’s disease. Neuroimage 49:2581–2587
Wajda DA, Mirelman A, Hausdorff JM, Sosnoff JJ (2017) Intervention modalities for targeting cognitive-motor interference in individuals with neurodegenerative disease: a systematic review. Expert Rev Neurother 17:251–261
Li Z, Wang T, Liu H, Jiang Y, Wang Z, Zhuang J (2020) Dual-task training on gait, motor symptoms, and balance in patients with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil 34:1355–1367
Radder DLM, Silva L, de Lima A, Domingos J, Keus SHJ, van Nimwegen M, Bloem BR, de Vries NM (2020) Physiotherapy in Parkinson’s disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair 34:871–880
Strouwen C, Molenaar E, Munks L, Keus SHJ, Bloem BR, Rochester L, Nieuwboer A (2015) Dual tasking in Parkinson’s disease: should we train hazardous behavior? Expert Rev Neurother 15:1031–1039
Domellöf ME, Ekman U, Forsgren L, Elgh E (2015) Cognitive function in the early phase of Parkinson’s disease, a five-year follow-up. Acta Neurol Scand 132:79–88
Zhang WS, Gao C, Tan YY, Chen SD (2021) Prevalence of freezing of gait in Parkinson’s disease: a systematic review and meta-analysis. J Neurol 268:4138–4150
Cosgrove J, Alty JE, Jamieson S (2015) Cognitive impairment in Parkinson’s disease. Postgrad Med J 91:212–220
Ali N, Tian H, Thabane L, Ma J, Wu H, Zhong Q, Gao Y, Sun C, Zhu Y, Wang T (2022) The effects of dual-task training on cognitive and physical functions in older adults with cognitive impairment; a systematic review and meta-analysis. J Prev Alzheimers Dis 9:359–370
Li Q, Gong B, Zhao Y, Wu C (2022) Effect of exercise cognitive combined training on physical function in cognitively healthy older adults: a systematic review and meta-analysis. J Aging Phys Act 1:1–16
Rieker JA, Reales JM, Muiños M, Ballesteros S (2022) The effects of combined cognitive-physical interventions on cognitive functioning in healthy older adults: a systematic review and multilevel meta-analysis. Front Hum Neurosci 16:838968
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5:210
Maidan I, Nieuwhof F, Bernad-Elazari H, Bloem BR, Giladi N, Hausdorff JM, Claassen J, Mirelman A (2018) Evidence for differential effects of 2 forms of exercise on prefrontal plasticity during walking in Parkinson’s disease. Neurorehabil Neural Repair 32:200–208
Pompeu JE, Mendes FA, Silva KG, Lobo AM, Oliveira Tde P, Zomignani AP, Piemonte ME (2012) Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy 98:196–204
King LA, Mancini M, Smulders K, Harker G, Lapidus JA, Ramsey K, Carlson-Kuhta P, Fling BW, Nutt JG, Peterson DS, Horak FB (2020) Cognitively challenging agility boot camp program for freezing of gait in Parkinson disease. Neurorehabil Neural Repair. https://doi.org/10.1177/1545968320909331
Jung SH, Hasegawa N, Mancini M, King LA, Carlson-Kuhta P, Smulders K, Peterson DS, Barlow N, Harker G, Morris R, Lapidus J, Nutt JG, Horak FB (2020) Effects of the agility boot camp with cognitive challenge (ABC-C) exercise program for Parkinson’s disease. npj Parkinsons Dis 6:8
Hasegawa N, Shah VV, Harker G, Carlson-Kuhta P, Nutt JG, Lapidus JA, Jung SH, Barlow N, King LA, Horak FB, Mancini M (2020) Responsiveness of objective vs. clinical balance domain outcomes for exercise intervention in Parkinson’s disease. Front Neurol 11:940
Conradsson D, Lofgren N, Nero H, Hagstromer M, Stahle A, Lokk J, Franzen E (2015) The effects of highly challenging balance training in elderly with Parkinson’s disease: a randomized controlled trial. Neurorehabil Neural Repair 29:827–836
Wallen MB, Hagstromer M, Conradsson D, Sorjonen K, Franzen E (2018) Long-term effects of highly challenging balance training in Parkinson’s disease-a randomized controlled trial. Clin Rehabil. https://doi.org/10.1177/0269215518784338
Strouwen C, Molenaar E, Munks L, Keus SHJ, Zijlmans JCM, Vandenberghe W, Bloem BR, Nieuwboer A (2017) Training dual tasks together or apart in Parkinson’s disease: results from the DUALITY trial. Mov Disord 32:1201–1210
Review Manager (RevMan) (2020) In: The Cochrane Collaboration
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) (2022) Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane. Available from www.training.cochrane.org/handbook
GRADEpro GDT: GRADEpro Guideline Development Tool [Software] (2022) In: McMaster University and Evidence Prime
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Online) 372:n71–n71
Geroin C, Nonnekes J, de Vries NM, Strouwen C, Smania N, Tinazzi M, Nieuwboer A, Bloem BR (2018) Does dual-task training improve spatiotemporal gait parameters in Parkinson’s disease? Parkinsonism Relat Disord 55:86–91
Strouwen C, Molenaar E, Munks L, Broeder S, Ginis P, Bloem BR, Nieuwboer A, Heremans E (2019) Determinants of dual-task training effect size in Parkinson disease: who will benefit most? J Neurol Phys Ther 43:3–11
Pohl P, Wressle E, Lundin F, Enthoven P, Dizdar N (2020) Group-based music intervention in Parkinson’s disease—findings from a mixed-methods study. Clin Rehabil 34:533–544
Yang YR, Cheng SJ, Lee YJ, Liu YC, Wang RY (2019) Cognitive and motor dual task gait training exerted specific training effects on dual task gait performance in individuals with Parkinson’s disease: a randomized controlled pilot study. PLoS ONE 14:e0218180
Johansson H, Freidle M, Ekman U, Schalling E, Leavy B, Svenningsson P, Hagstromer M, Franzen E (2020) Feasibility aspects of exploring exercise-induced neuroplasticity in Parkinson’s disease: a pilot randomized controlled trial. Parkinsons Dis 2020:2410863
Lofgren N, Conradsson D, Rennie L, Moe-Nilssen R, Franzen E (2019) The Effects of integrated single- and dual-task training on automaticity and attention allocation in parkinson’s disease: a secondary analysis from a randomized trial. Neuropsychology 33:147–156
Rosenfeldt AB, Penko AL, Streicher MC, Zimmerman NM, Koop MM, Alberts JL (2019) Improvements in temporal and postural aspects of gait vary following single- and multi-modal training in individuals with Parkinson’s disease. Parkinsonism Relat Disord 64:280–285
San Martín Valenzuela C, Moscardó LD, López-Pascual J, Serra-Añó P, Tomás JM (2020) Effects of dual-task group training on gait, cognitive executive function, and quality of life in people with parkinson disease: results of randomized controlled DUALGAIT trial. Arch Phys Med Rehabil 101:1849-1856.e1841
Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH (2011) Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther 91:862–874
Fritz S, Lusardi M (2009) White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther 32:46–49
Hass CJ, Bishop M, Moscovich M, Stegemoller EL, Skinner J, Malaty IA, Wagle Shukla A, McFarland N, Okun MS (2014) Defining the clinically meaningful difference in gait speed in persons with Parkinson disease. J Neurol Phys Ther 38:233–238
Strouwen C, Molenaar E, Keus SHJ, Munks L, Heremans E, Vandenberghe W, Bloem BR, Nieuwboer A (2016) Are factors related to dual-task performance in people with Parkinson’s disease dependent on the type of dual task? Parkinsonism Relat Disord 23:23–30
Christofoletti G, McNeely ME, Campbell MC, Duncan RP, Earhart GM (2016) Investigation of factors impacting mobility and gait in Parkinson disease. Hum Mov Sci 49:308–314
Gilat M, Bell PT, Martens KAE, Georgiades MJ, Hall JM, Walton CC, Lewis SJG, Shine JM (2017) Dopamine depletion impairs gait automaticity by altering cortico-striatal and cerebellar processing in Parkinson’s disease. Neuroimage 152:207–220
Henderson EJ, Lord SR, Brodie MA, Gaunt DM, Lawrence AD, Close JC, Whone AL, Ben-Shlomo Y (2016) Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 15:249–258
Conradsson D, Leavy B, Hagstromer M, Nilsson MH, Franzen E (2017) Physiotherapy for Parkinson’s disease in Sweden: provision, expertise, and multi-professional collaborations. Mov Disorders Clin Pract 4:843–851
Smulders K, Esselink RA, Weiss A, Kessels RP, Geurts AC, Bloem BR (2012) Assessment of dual tasking has no clinical value for fall prediction in Parkinson’s disease. J Neurol 259:1840–1847
Vance RC, Healy DG, Galvin R, French HP (2015) Dual tasking with the timed “up & go” test improves detection of risk of falls in people with Parkinson disease. Phys Ther 95:95–102
Lofgren N, Conradsson D, Joseph C, Leavy B, Hagstromer M, Franzen E (2019) Factors associated with responsiveness to gait and balance training in people with Parkinson disease. J Neurol Phys Ther 43:42–49
Chung YC, Fisher BE, Finley JM, Kim A, Petkus AJ, Schiehser DM, Jakowec MW, Petzinger GM (2021) Cognition and motor learning in a Parkinson’s disease cohort: importance of recall in episodic memory. NeuroReport 32:1153–1160
Strouwen C, Molenaar E, Keus SHJ, Munks L, Bloem BR, Nieuwboer A (2016) Test-retest reliability of dual-task outcome measures in people with Parkinson disease. Phys Ther 96:1276–1286
Acknowledgements
We wish to thank information specialists/librarians Sabina Gillsund and Love Strandberg at Karolinska Institutet Library for their help with literature searches.
Funding
Open access funding provided by Karolinska Institute. Authors BL and HJ were supported by grants from the Kamprad family foundation, Region Stockholm, the Swedish Parkinson Foundation, and the Stockholm Sjukhem Foundation. Authors AKF and EK were financed by budget resources of the involved departments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors BL, HJ and IH: Declarations of interest: none. Author AKF has received grants from the German Parkinson Society and the German Alzheimer’s Society, as well as honoraria from Springer Medizin Verlag GmbH, Heidelberg, Germany; Springer-Verlag GmbH, Berlin; ProLog Wissen GmbH, Cologne, Germany; pro audito Switzerland, Zürich, Switzerland; Seminar- und Fortbildungszentrum Rheine, Germany; and LOGOMANIA, Fendt & Sax GbR, Munich, Germany. AFK is author of the cognitive intervention programs “NEUROvitalis” but receives no corresponding honoraria. Author EK has received grants from the German Ministry of Education and Research, ParkinsonFonds Deutschland gGmbH, the German Parkinson Society, and the German Alzheimer’s Society, and honoraria from Oticon GmbH, Hamburg, Germany; Lilly Pharma GmbH, Bad Homburg, Germany; Bernafon AG, Bern, Switzerland; and Desitin GmbH, Hamburg, Germany. EK is author of the cognitive intervention programs “NEUROvitalis” but receives no corresponding honoraria.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johansson, H., Folkerts, AK., Hammarström, I. et al. Effects of motor–cognitive training on dual-task performance in people with Parkinson’s disease: a systematic review and meta-analysis. J Neurol 270, 2890–2907 (2023). https://doi.org/10.1007/s00415-023-11610-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11610-8