Abstract
Background
Soma and neurite density imaging (SANDI) is a new biophysical model that incorporates soma in addition to neurite density, thus possibly providing more specific information about the complex pathological processes of multiple sclerosis (MS).
Purpose
To discriminate the pathological abnormalities of MS white matter (WM) lesions, normal-appearing (NA) WM and cortex and to evaluate the associations among SANDI-derived measures, clinical disability, and conventional MRI variables.
Methods
Twenty healthy controls (HC) and 23 MS underwent a 3 T brain MRI. Using SANDI on diffusion-weighted sequence, the fractions of neurite (fneurite) and soma (fsoma) were assessed in WM lesions, NAWM, and cortex.
Results
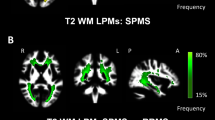
Compared to HC WM, MS NAWM showed lower fneurite (false discovery rate [FDR]-p = 0.011). In MS patients, WM lesions showed lower fneurite and fsoma compared to both HC and MS NAWM (FDR-p < 0.001 for all). In the cortex, MS patients had lower fneurite and fsoma compared to HC (FDR-p ≤ 0.009). Compared to both HC and RRMS, PMS patients had lower fneurite in NAWM (vs HC: FDR-p < 0.001; vs RRMS: FDR-p = 0.003) and cortex (vs HC: FDR-p < 0.001; vs RRMS: p = 0.031, not surviving FDR correction), and lower cortical fsoma (vs HC: FDR-p < 0.001; vs RRMS: FDR-p = 0.009). Compared to HC, PMS also showed a higher fsoma in NAWM (FDR-p = 0.015). Fneurite and fsoma in the different brain compartments were correlated with age, phenotype, disease duration, disability, WM lesion volumes, normalized brain, cortical, and WM volumes (r from − 0.761 to 0.821, FDR-p ≤ 0.4).
Conclusions
SANDI may represent a clinically relevant model to discriminate different neurodegenerative phenomena that gradually accumulate through MS disease course.




Similar content being viewed by others
Data availability
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
Abbreviations
- DMT:
-
Disease-modifying treatment
- EDSS:
-
Expanded disability status scale
- NBV:
-
Normalized brain volume
- NCV:
-
Normalized cortical volume
- NWMV:
-
Normalized white matter volume
- SANDI:
-
Soma and neurite density imaging
References
Alexander DC, Dyrby TB, Nilsson M, Zhang H (2019) Imaging brain microstructure with diffusion MRI: practicality and applications. NMR Biomed 32:e3841
Andersson JLR, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063–1078
Carassiti D, Altmann DR, Petrova N, Pakkenberg B, Scaravilli F, Schmierer K (2018) Neuronal loss, demyelination and volume change in the multiple sclerosis neocortex. Neuropathol Appl Neurobiol 44:377–390
Cordero-Grande L, Christiaens D, Hutter J, Price AN, Hajnal JV (2019) Complex diffusion-weighted image estimation via matrix recovery under general noise models. Neuroimage 200:391–404
Daducci A, Canales-Rodriguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran JP (2015) Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. Neuroimage 105:32–44
Eshaghi A, Marinescu RV, Young AL, Firth NC, Prados F, Jorge Cardoso M, Tur C, De Angelis F, Cawley N, Brownlee WJ, De Stefano N, Laura Stromillo M, Battaglini M, Ruggieri S, Gasperini C, Filippi M, Rocca MA, Rovira A, Sastre-Garriga J, Geurts JJG, Vrenken H, Wottschel V, Leurs CE, Uitdehaag B, Pirpamer L, Enzinger C, Ourselin S, Gandini Wheeler-Kingshott CA, Chard D, Thompson AJ, Barkhof F, Alexander DC, Ciccarelli O (2018) Progression of regional grey matter atrophy in multiple sclerosis. Brain 141:1665–1677
Fieremans E, Jensen JH, Helpern JA (2011) White matter characterization with diffusional kurtosis imaging. Neuroimage 58:177–188
Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca MA (2018) Multiple sclerosis. Nat Rev Dis Primers 4:43
Filippi M, Bruck W, Chard D, Fazekas F, Geurts JJG, Enzinger C, Hametner S, Kuhlmann T, Preziosa P, Rovira A, Schmierer K, Stadelmann C, Rocca MA, Attendees of the Correlation between P, workshop MRIfiM (2019) Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol 18:198–210
Filippi M, Preziosa P, Langdon D, Lassmann H, Paul F, Rovira A, Schoonheim MM, Solari A, Stankoff B, Rocca MA (2020) Identifying progression in multiple sclerosis: new perspectives. Ann Neurol 88:438–452
Filippi M, Preziosa P, Rocca MA (2017) Microstructural MR imaging techniques in multiple sclerosis. Neuroimaging Clin N Am 27:313–333
Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, Mandrekar J, Bramow S, Metz I, Bruck W, Lassmann H, Lucchinetti CF (2015) Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 78:710–721
Granziera C, Wuerfel J, Barkhof F, Calabrese M, De Stefano N, Enzinger C, Evangelou N, Filippi M, Geurts JJG, Reich DS, Rocca MA, Ropele S, Rovira A, Sati P, Toosy AT, Vrenken H, Gandini Wheeler-Kingshott CAM, Kappos L, Group MS (2021) Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain 144:1296–1311
Ianus A, Carvalho J, Fernandes FF, Cruz R, Chavarrias C, Palombo M, Shemesh N (2022) Soma and Neurite Density MRI (SANDI) of the in-vivo mouse brain and comparison with the Allen Brain Atlas. Neuroimage 254:119135
Jespersen SN, Kroenke CD, Ostergaard L, Ackerman JJ, Yablonskiy DA (2007) Modeling dendrite density from magnetic resonance diffusion measurements. Neuroimage 34:1473–1486
Kaden E, Kruggel F, Alexander DC (2016) Quantitative mapping of the per-axon diffusion coefficients in brain white matter. Magn Reson Med 75:1752–1763
Kellner E, Dhital B, Kiselev VG, Reisert M (2016) Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med 76:1574–1581
Klaver R, Popescu V, Voorn P, Galis-de Graaf Y, van der Valk P, de Vries HE, Schenk GJ, Geurts JJ (2015) Neuronal and axonal loss in normal-appearing gray matter and subpial lesions in multiple sclerosis. J Neuropathol Exp Neurol 74:453–458
Kuhlmann T, Ludwin S, Prat A, Antel J, Bruck W, Lassmann H (2017) An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol 133:13–24
Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712
Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I (2018) Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol 135:511–528
Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, Aloisi F, Reynolds R (2010) A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 68:477–493
Mahad DH, Trapp BD, Lassmann H (2015) Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 14:183–193
Novikov DS, Fieremans E, Jespersen SN, Kiselev VG (2019) Quantifying brain microstructure with diffusion MRI: theory and parameter estimation. NMR Biomed 32:e3998
Olesen JL, Ostergaard L, Shemesh N, Jespersen SN (2022) Diffusion time dependence, power-law scaling, and exchange in gray matter. Neuroimage 251:118976
Palombo M, Grussu F, Torben Schneider T, C. DG, Alexander DCGWKCAM, Zhang H (2020) New potential MRI markers of glial scarring and tissue damage in multiple sclerosis spinal cord pathology using diffusion MRI. In: Proc. Intl. Soc. Mag. Reson. Med.
Palombo M, Ianus A, Guerreri M, Nunes D, Alexander DC, Shemesh N, Zhang H (2020) SANDI: A compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI. Neuroimage 215:116835
Peterson JW, Bo L, Mork S, Chang A, Trapp BD (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400
Popescu V, Klaver R, Voorn P, Galis-de Graaf Y, Knol DL, Twisk JW, Versteeg A, Schenk GJ, Van der Valk P, Barkhof F, De Vries HE, Vrenken H, Geurts JJ (2015) What drives MRI-measured cortical atrophy in multiple sclerosis? Mult Scler 21:1280–1290
Preziosa P, Kiljan S, Steenwijk MD, Meani A, van de Berg WDJ, Schenk GJ, Rocca MA, Filippi M, Geurts JJG, Jonkman LE (2019) Axonal degeneration as substrate of fractional anisotropy abnormalities in multiple sclerosis cortex. Brain 142:1921–1937
Preziosa P, Pagani E, Moiola L, Rodegher M, Filippi M, Rocca MA (2021) Occurrence and microstructural features of slowly expanding lesions on fingolimod or natalizumab treatment in multiple sclerosis. Mult Scler 27:1520–1532
Preziosa P, Rocca MA, Filippi M (2021) Central vein sign and iron rim in multiple sclerosis: ready for clinical use? Curr Opin Neurol 34:505–513
Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh CH, Connelly A (2019) MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202:116137
Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320
Valverde S, Cabezas M, Roura E, Gonzalez-Villa S, Pareto D, Vilanova JC, Ramio-Torrenta L, Rovira A, Oliver A, Llado X (2017) Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage 155:159–168
Veraart J, Fieremans E, Novikov DS (2016) Diffusion MRI noise mapping using random matrix theory. Magn Reson Med 76:1582–1593
Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM (2006) Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 67:960–967
Yang DM, Huettner JE, Bretthorst GL, Neil JJ, Garbow JR, Ackerman JJH (2018) Intracellular water preexchange lifetime in neurons and astrocytes. Magn Reson Med 79:1616–1627
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61:1000–1016
Funding
MP is supported by UKRI Future Leaders Fellowship grant no. MR/T020296/2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Margoni reports grants and personal fees from Almiral. She was awarded a MAGNIMS-ECTRIMS fellowship in 2020. E. Pagani received speakers’ honoraria from Biogen Idec. P. Preziosa received speaker honoraria from Roche, Biogen, Novartis, Merck Serono, Bristol Myers Squibb and Genzyme. He has received research support from Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. M. Palombo, M. Azzimonti and M. Gueye have nothing to disclose. M. Filippi is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Associate Editor of Radiology, and Associate Editor of Neurological Sciences; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, Takeda; scientific direction of educational evens for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA).M.A. Rocca received speakers’ honoraria from Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva, and receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla.
Ethical statement
Approval was obtained from the institutional ethical standards committee on human experimentation of IRCCS Ospedale San Raffaele for any experiments using human subjects (protocol number 2015–33). Written informed consent was obtained from all subjects prior to study participation according to the Declaration of Helsinki.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Margoni, M., Pagani, E., Preziosa, P. et al. In vivo quantification of brain soma and neurite density abnormalities in multiple sclerosis. J Neurol 270, 433–445 (2023). https://doi.org/10.1007/s00415-022-11386-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11386-3