Abstract
Background
White matter (WM) atrophy is relevant in multiple sclerosis (MS), but the methods of analysis currently used are not specific for microstructural changes. The aims of this study were to assess the use of advanced diffusion-weighted imaging (DWI) techniques proposed as measures of baseline and longitudinal WM atrophy in MS and to analyze whether these measures helped explain MS clinical disability (including cognitive impairment) better than volumetric and diffusion tensor (DT)-derived measures.
Methods
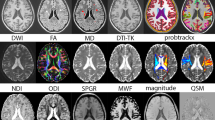
3DT1-weighted and DWI sequences were applied to 86 MS and 55 healthy controls (HC) at baseline and after one-year. Intra-cellular volume (vic) maps were computed from neurite orientation dispersion and density imaging model. Voxel-wise fiber-bundle cross-section (FCS) atrophy in MS compared to HC was estimated. Maps of fractional anisotropy and mean diffusivity were also obtained from DWI for a comparison with the proposed advanced DW-derived measures (vic and FCS).
Results
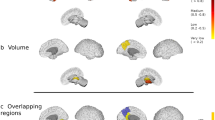
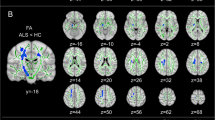
Both at baseline and after 1-year, only FCS measure showed a significant atrophy in relapsing–remitting (RR) MS compared to HC and in progressive MS compared to RRMS, mainly located in specific WM tracts (corticospinal tract, splenium of the corpus callosum, left optic radiation, bilateral cingulum, middle cerebellar peduncle and anterior commissure, p value < 0.05). Global baseline FCS and vic were the selected predictors of clinical (R-sq = 0.33, p = 0.007) and cognitive scores (R-sq = 0.29, p = 0.0014) in a linear regression model.
Conclusion
Voxel-based FCS was able to detect WM tracts atrophy in MS clinical phenotypes with greater anatomical specificity compared to other measures (volumetric and DT-derived measures of WM damage). FCS and vic measured at baseline in the WM were the best predictors of clinical disability and cognitive impairment.




Similar content being viewed by others
References
Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J (2014) Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 103:411–426. https://doi.org/10.1016/j.neuroimage.2014.07.061 (PubMed PMID: 25109526)
Roine T, Jeurissen B, Perrone D, Aelterman J, Philips W, Leemans A et al (2015) Informed constrained spherical deconvolution (iCSD). Med Image Anal 24(1):269–281. https://doi.org/10.1016/j.media.2015.01.001 (Epub 2015/02/11. PubMed PMID: 25660002)
Tournier JD, Calamante F, Gadian DG, Connelly A (2004) Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage 23(3):1176–1185. https://doi.org/10.1016/j.neuroimage.2004.07.037 (PubMed PMID: 15528117)
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61(4):1000–1016. https://doi.org/10.1016/j.neuroimage.2012.03.072 (PubMed PMID: 22484410)
Grussu F, Schneider T, Tur C, Yates RL, Tachrount M, Ianus A et al (2017) Neurite dispersion: a new marker of multiple sclerosis spinal cord pathology? Ann Clin Transl Neurol 4(9):663–679. https://doi.org/10.1002/acn3.445 (PubMed PMID: 28904988; PubMed Central PMCID: PMCPMC5590517)
Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR et al (2017) Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 144(Pt A):58–73. https://doi.org/10.1016/j.neuroimage.2016.09.029 (PubMed PMID: 27639350; PubMed Central PMCID: PMCPMC5182031)
Caverzasi E, Papinutto N, Castellano A, Zhu AH, Scifo P, Riva M et al (2016) Neurite orientation dispersion and density imaging color maps to characterize brain diffusion in neurologic disorders. J Neuroimag 26(5):494–498. https://doi.org/10.1111/jon.12359 (PubMed PMID: 27214558)
Myrte Strik LECL, Shanahan CJ, van der Walt A, Boonstra FMC, Glarin R, Kilpatrick TJ, Geurts JJG, Cleary JO, Schoonheim MM, Galea MP, Kolbe SC (2021) Axonal loss in major sensorimotor tracts is associated with impaired motor performance in minimally disabled multiple sclerosis patients. Brain Commun. https://doi.org/10.1093/braincomms/fcab032
Collorone S, Cawley N, Grussu F, Prados F, Tona F, Calvi A et al (2020) Reduced neurite density in the brain and cervical spinal cord in relapsing-remitting multiple sclerosis: a NODDI study. Mult Scler 26(13):1647–1657. https://doi.org/10.1177/1352458519885107 (Epub 2019/11/05. PubMed PMID: 31682198)
Collorone S, Prados F, Kanber B, Cawley NM, Tur C, Grussu F et al (2021) Brain microstructural and metabolic alterations detected in vivo at onset of the first demyelinating event. Brain. https://doi.org/10.1093/brain/awab043 (Epub 2021/04/28. PubMed PMID: 33903905)
Granberg T, Fan Q, Treaba CA, Ouellette R, Herranz E, Mangeat G et al (2017) In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain 140(11):2912–2926. https://doi.org/10.1093/brain/awx247 (Epub 2017/10/21. PubMed PMID: 29053798; PubMed Central PMCID: PMCPMC5841207)
Rahmanzadeh R, Lu PJ, Barakovic M, Weigel M, Maggi P, Nguyen TD et al (2021) Myelin and axon pathology in multiple sclerosis assessed by myelin water and multi-shell diffusion imaging. Brain. https://doi.org/10.1093/brain/awab088 (Epub 2021/03/12. PubMed PMID: 33693571)
Sacco S, Caverzasi E, Papinutto N, Cordano C, Bischof A, Gundel T et al (2020) Neurite orientation dispersion and density imaging for assessing acute inflammation and lesion evolution in MS. AJNR Am J Neuroradiol 41(12):2219–2226. https://doi.org/10.3174/ajnr.A6862 (Epub 2020/11/07. PubMed PMID: 33154077; PubMed Central PMCID: PMCPMC7963254)
Spano B, Giulietti G, Pisani V, Morreale M, Tuzzi E, Nocentini U et al (2018) Disruption of neurite morphology parallels MS progression. Neurol Neuroimmunol Neuroinflamm. 5(6):e502. https://doi.org/10.1212/NXI.0000000000000502 (Epub 2018/10/23. PubMed PMID: 30345330; PubMed Central PMCID: PMCPMC6192688)
Gajamange S, Raffelt D, Dhollander T, Lui E, van der Walt A, Kilpatrick T et al (2018) Fibre-specific white matter changes in multiple sclerosis patients with optic neuritis. Neuroimage Clin. 17:60–68. https://doi.org/10.1016/j.nicl.2017.09.027 (Epub 2018/03/13. PubMed PMID: 29527473; PubMed Central PMCID: PMCPMC5842545)
Carandini T, Mancini M, Bogdan I, Rae CL, Barritt AW, Sethi A et al (2021) Disruption of brainstem monoaminergic fibre tracts in multiple sclerosis as a putative mechanism for cognitive fatigue: a fixel-based analysis. Neuroimage Clin. 30:102587. https://doi.org/10.1016/j.nicl.2021.102587 (Epub 2021/02/21. PubMed PMID: 33610097; PubMed Central PMCID: PMCPMC7903010)
Storelli L, Pagani E, Preziosa P, Filippi M, Rocca MA (2020) Measurement of white matter fiber-bundle cross-section in multiple sclerosis using diffusion-weighted imaging. Mult Scler. https://doi.org/10.1177/1352458520938999 (Epub 2020/07/15. PubMed PMID: 32662738)
De Santis S, Bastiani M, Droby A, Kolber P, Zipp F, Pracht E et al (2019) Characterizing microstructural tissue properties in multiple sclerosis with diffusion MRI at 7T and 3T: the impact of the experimental design. Neuroscience 403:17–26. https://doi.org/10.1016/j.neuroscience.2018.03.048 (Epub 2018/04/10. PubMed PMID: 29631021)
Schneider T, Brownlee W, Zhang H, Ciccarelli O, Miller DH, Wheeler-Kingshott CG (2017) Sensitivity of multi-shell NODDI to multiple sclerosis white matter changes: a pilot study. Funct Neurol 32(2):97–101. https://doi.org/10.11138/fneur/2017.32.2.097 (Epub 2017/07/06. PubMed PMID: 28676143; PubMed Central PMCID: PMCPMC5507159)
Damjanovic D, Valsasina P, Rocca MA, Stromillo ML, Gallo A, Enzinger C et al (2017) Hippocampal and deep gray matter nuclei atrophy is relevant for explaining cognitive impairment in MS: a multicenter study. AJNR Am J Neuroradiol 38(1):18–24. https://doi.org/10.3174/ajnr.A4952 (PubMed PMID: 27686487)
Roosendaal SD, Bendfeldt K, Vrenken H, Polman CH, Borgwardt S, Radue EW et al (2011) Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult Scler 17(9):1098–1106. https://doi.org/10.1177/1352458511404916 (Epub 2011/05/19. PubMed PMID: 21586487)
Fields RD (2010) Neuroscience. Change in the brain’s white matter. Science 330(6005):768–769. https://doi.org/10.1126/science.1199139 (PubMed PMID: 21051624; PubMed Central PMCID: PMCPMC3201847)
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173. https://doi.org/10.1016/S1474-4422(17)30470-2 (PubMed PMID: 29275977)
Valverde S, Cabezas M, Roura E, Gonzalez-Villa S, Pareto D, Vilanova JC et al (2017) Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage 155:159–168. https://doi.org/10.1016/j.neuroimage.2017.04.034 (PubMed PMID: WOS:000405460900013)
Battaglini M, Jenkinson M, De Stefano N (2011) Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp. https://doi.org/10.1002/hbm.21344 (Epub 2011/09/02. PubMed PMID: 21882300)
Smith SM, De Stefano N, Jenkinson M, Matthews PM (2001) Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr 25(3):466–475 (Epub 2001/05/15. PubMed PMID: 11351200)
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A et al (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17(1):479–489 (Epub 2002/12/17. PubMed PMID: 12482100)
Andersson JLR, Graham MS, Drobnjak I, Zhang H, Filippini N, Bastiani M (2017) Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage 152:450–466. https://doi.org/10.1016/j.neuroimage.2017.02.085 (PubMed PMID: 28284799; PubMed Central PMCID: PMCPMC5445723)
Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S et al (2003) Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50(5):1077–1088. https://doi.org/10.1002/mrm.10609 (Epub 2003/10/31. PubMed PMID: 14587019)
Chung AW, Seunarine KK, Clark CA (2016) NODDI reproducibility and variability with magnetic field strength: a comparison between 1.5 T and 3 T. Hum Brain Mapp 37(12):4550–4565. https://doi.org/10.1002/hbm.23328 (PubMed PMID: WOS:000387399200024)
Tax CMW, Jeurissen B, Vos SB, Viergever MA, Leemans A (2014) Recursive calibration of the fiber response function for spherical deconvolution of diffusion MRI data. Neuroimage 86:67–80. https://doi.org/10.1016/j.neuroimage.2013.07.067 (PubMed PMID: WOS:000330335300009)
Tournier JD, Calamante F, Connelly A (2007) Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35(4):1459–1472. https://doi.org/10.1016/j.neuroimage.2007.02.016 (PubMed PMID: 17379540)
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11(6 Pt 1):805–821. https://doi.org/10.1006/nimg.2000.0582 (Epub 2000/06/22. PubMed PMID: 10860804)
Cree BAC, Cutter G, Wolinsky JS, Freedman MS, Comi G, Giovannoni G et al (2020) Safety and efficacy of MD1003 (high-dose biotin) in patients with progressive multiple sclerosis (SPI2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 19(12):988–997. https://doi.org/10.1016/S1474-4422(20)30347-1 (Epub 2020/11/24. PubMed PMID: 33222767)
Kappos L, Wiendl H, Selmaj K, Arnold DL, Havrdova E, Boyko A et al (2015) Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 373(15):1418–1428. https://doi.org/10.1056/NEJMoa1501481 (Epub 2015/10/09. PubMed PMID: 26444729)
Amato MP, Morra VB, Falautano M, Ghezzi A, Goretti B, Patti F et al (2019) Correction to: Cognitive assessment in multiple sclerosis-an Italian consensus. Neurol Sci 40(5):1097. https://doi.org/10.1007/s10072-019-03852-0 (Epub 2019/03/23. PubMed PMID: 30900096)
Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R et al (2017) Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 23(5):721–733. https://doi.org/10.1177/1352458517690821 (Epub 2017/02/17. PubMed PMID: 28206827; PubMed Central PMCID: PMCPMC5405816)
Morrow SA, O’Connor PW, Polman CH, Goodman AD, Kappos L, Lublin FD et al (2010) Evaluation of the symbol digit modalities test (SDMT) and MS neuropsychological screening questionnaire (MSNQ) in natalizumab-treated MS patients over 48 weeks. Mult Scler 16(11):1385–1392. https://doi.org/10.1177/1352458510378021 (PubMed PMID: 20739335)
Benedict RHB, Cookfair D, Gavett R, Gunther M, Munschauer F, Garg N et al (2006) Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 12:549–558
Rao SM, Martin AL, Huelin R, Wissinger E, Khankhel Z, Kim E et al (2014) Correlations between MRI and information processing speed in MS: a meta-analysis. Mult Scler Int 2014:975803. https://doi.org/10.1155/2014/975803 (PubMed PMID: 24795824; PubMed Central PMCID: PMCPMC3984845)
Andersen KW, Lundell H, Nilsson M, Topgaard D, Sellebjerg F, Szczepankiewicz F, Siebner HR, Blinkenberg M, Dyrby TB (2020) Disentangling white-matter damage from physiological fibre orientation dispersion in multiple sclerosis. Brain Commun. https://doi.org/10.1093/braincomms/fcaa077
Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4(3):316–329. https://doi.org/10.1016/j.nurt.2007.05.011 (PubMed PMID: 17599699; PubMed Central PMCID: PMCPMC2041910)
Raffelt DA, Smith RE, Ridgway GR, Tournier JD, Vaughan DN, Rose S et al (2015) Connectivity-based fixel enhancement: whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage 117:40–55. https://doi.org/10.1016/j.neuroimage.2015.05.039 (PubMed PMID: 26004503; PubMed Central PMCID: PMCPMC4528070)
Storelli L, Rocca MA, Pagani E, Van Hecke W, Horsfield MA, De Stefano N et al (2018) Measurement of whole-brain and gray matter atrophy in multiple sclerosis: assessment with MR imaging. Radiology 288(2):554–564. https://doi.org/10.1148/radiol.2018172468 (PubMed PMID: 29714673)
Amato M, Morra V, Falautano M, Ghezzi A, Goretti B, Patti F et al (2018) Cognitive assessment in multiple sclerosis-an Italian consensus. Neurol Sci 39(8):1317–1324. https://doi.org/10.1007/s10072-018-3427-x (PubMed PMID: WOS:000439465800001)
Pardini M, Uccelli A, Grafman J, Yaldizli O, Mancardi G, Roccatagliata L (2014) Isolated cognitive relapses in multiple sclerosis. J Neurol Neurosur Ps 85(9):1035–1037. https://doi.org/10.1136/jnnp-2013-307275 (PubMed PMID: WOS:000342430400020)
Acknowledgements
This study was partially supported by Fondazione Italiana Sclerosi Multipla with a research fellowship (FISM 2019/BR/009) and a research Grant (FISM2018/R/16), and financed or co-financed with the ‘5 per mille’ public funding.
Author information
Authors and Affiliations
Contributions
LS, EP, MF and MAR contributed to the conception and design of the study. LS, EP, AM, PP contributed to the acquisition and analysis of data. LS, EP, AM, PP contributed to drafting the text and preparing the figures. All the authors contributed to revising the manuscript and gave their approval to its current version.
Corresponding author
Ethics declarations
Conflicts of interest
L. Storelli, E. Pagani, and A. Meani have nothing to disclose. P. Preziosa received speaker honoraria from Biogen Idec, Novartis, Bristol Myers Squibb, Genzyme and ExceMED. He is supported by a senior research fellowship FISM—Fondazione Italiana Sclerosi Multipla—cod. 2019/BS/009 and financed or co-financed with the ‘5 per mille’ public funding. Prof. Filippi is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Associate Editor of Radiology, and Associate Editor of Neurological Sciences; received compensation for consulting services and/or speaking activities from Alexion, Almirall, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). Prof. M.A. Rocca received speaker honoraria from Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva, and receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla.
Ethical Standards
The ethics committee of IRCCS Ospedale San Raffaele approved the research protocol and the study was conducted in accordance with the principles of the Declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Storelli, L., Pagani, E., Meani, A. et al. Advanced diffusion-weighted imaging models better characterize white matter neurodegeneration and clinical outcomes in multiple sclerosis. J Neurol 269, 4729–4741 (2022). https://doi.org/10.1007/s00415-022-11104-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11104-z