Abstract
Objective
We investigated if anodal transcranial direct current stimulation (A-tDCS), applied over the supplementary motor areas (SMAs), could improve gait initiation in Parkinson’s disease (PD) with freezing of gait (FOG).
Methods
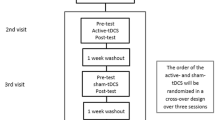
In this double-blinded cross-over pilot study, ten PD with FOG underwent two stimulation sessions: A-tDCS (1 mA, 10 min) and sham stimulation. Eight blocks of gait initiation were collected per session: (1) pre-tDCS, with acoustic cueing; (2) pre-tDCS, self-initiated (no cue); and (3–8) post-tDCS, self-initiated. Gait initiation kinetics were analyzed with two-way repeated measures ANOVAs for the effects of A-tDCS.
Results
A-tDCS did not significantly improve the magnitude or timing of anticipatory postural adjustments or the execution of the first step during self-initiated gait compared with baseline measures (p > .13). The lack of significant change was not due to an inability to generate functional APAs since external cueing markedly improved gait initiation (p < .01).
Conclusions
A single dose of A-tDCS over the SMAs did not improve self-initiated gait in PD and FOG. Alternative approaches using a different dose or cortical target are worthy of exploration since individuals demonstrated the capacity to improve.
Significance
Neuromodulation strategies tailored to facilitate SMA activity may be ineffective for the treatment of gait initiation impairment in people with PD and FOG.


Similar content being viewed by others
References
Giladi N, McMahon D, Przedborski S et al (1992) Motor blocks in Parkinson’s disease. Neurology 42:333–339
Rahman S, Griffin HJ, Quinn NP, Jahanshahi M (2008) Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord 23:1428–1434. https://doi.org/10.1002/mds.21667
Moore ST, MacDougall HG, Ondo WG (2008) Ambulatory monitoring of freezing of gait in Parkinson’s disease. J Neurosci Methods 167:340–348. https://doi.org/10.1016/j.jneumeth.2007.08.023
Elble RJ, Cousins R, Leffler K, Hughes L (1996) Gait initiation by patients with lower-half parkinsonism. Brain 119(Pt 5):1705–1716
Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA (1997) Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord 12:206–215. https://doi.org/10.1002/mds.870120211
Jacobs JV, Nutt JG, Carlson-Kuhta P et al (2009) Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol 215:334–341. https://doi.org/10.1016/j.expneurol.2008.10.019
Crenna P, Frigo C (1991) A motor programme for the initiation of forward-oriented movements in humans. J Physiol 437:635–653. https://doi.org/10.1113/jphysiol.1991.sp018616
Elble RJ, Moody C, Leffler K, Sinha R (1994) The initiation of normal walking. Mov Disord 9:139–146. https://doi.org/10.1002/mds.870090203
Callisaya ML, Blizzard L, Martin K, Srikanth VK (2016) Gait initiation time is associated with the risk of multiple falls—a population-based study. Gait Posture 49:19–24. https://doi.org/10.1016/j.gaitpost.2016.06.006
Nutt JG, Bloem BR, Giladi N et al (2011) Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 10:734–744. https://doi.org/10.1016/S1474-4422(11)70143-0
Alexander GE, Crutcher MD (1990) Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol 64:133–150
Cunnington R, Iansek R, Bradshaw JL, Phillips JG (1995) Movement-related potentials in Parkinson’s disease. Presence and predictability of temporal and spatial cues. Brain 118(Pt 4):935–950
Jahanshahi M, Jenkins IH, Brown RG et al (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118(Pt 4):913–933
Deiber MP, Honda M, Ibañez V et al (1999) Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81:3065–3077
Jenkins IH, Jahanshahi M, Jueptner M et al (2000) Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123 (Pt 6):1216–1228
Hugon M, Massion J, Wiesendanger M (1982) Anticipatory postural changes induced by active unloading and comparison with passive unloading in man. Pflügers Arch Eur J Physiol 393:292–296. https://doi.org/10.1007/BF00581412
Viallet F, Massion J, Massarino R, Khalil R (1992) Coordination between posture and movement in a bimanual load lifting task: putative role of a medial frontal region including the supplementary motor area. Exp Brain Res 88:674–684. https://doi.org/10.1007/BF00228197
Jacobs JV, Lou JS, Kraakevik JA, Horak FB (2009) The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience 164:877–885. https://doi.org/10.1016/j.neuroscience.2009.08.002
Sabatini U, Boulanouar K, Fabre N et al (2000) Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 123(Pt 2):394–403
Fling BW, Cohen RG, Mancini M et al (2013) Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 136:2405–2418. https://doi.org/10.1093/brain/awt172
Fling BW, Cohen RG, Mancini M et al (2014) Functional reorganization of the locomotor network in parkinson patients with freezing of gait. PLoS One 9:e100291. https://doi.org/10.1371/journal.pone.0100291.s001
Schlenstedt C, Mancini M, Nutt J et al (2018) Are hypometric anticipatory postural adjustments contributing to freezing of gait in Parkinson’s disease? Front Aging Neurosci 10:36. https://doi.org/10.3389/fnagi.2018.00036
Lu C, Amundsen Huffmaster SL, Tuite PJ et al (2017) Effect of cue timing and modality on gait initiation in Parkinson disease with freezing of gait. Arch Phys Med Rehabil 98:1291–1299. https://doi.org/10.1016/j.apmr.2017.01.009
Vollmann H, Conde V, Sewerin S et al (2013) Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimul 6:101–107. https://doi.org/10.1016/j.brs.2012.03.018
Carter MJ, Maslovat D, Carlsen AN (2014) Anodal transcranial direct current stimulation applied over the supplementary motor area delays spontaneous anti-phase to in-phase transitions. J Neurophysiol. https://doi.org/10.1152/jn.00662.2014
Carlsen AN, Eagles JS, MacKinnon CD (2015) Transcranial direct current stimulation over the supplementary motor area modulates the preparatory activation level in the human motor system. Behav Brain Res 279:68–75. https://doi.org/10.1016/j.bbr.2014.11.009
Hayduk-Costa G, Drummond NM, Carlsen AN (2013) Anodal tDCS over SMA decreases the probability of withholding an anticipated action. Behav Brain Res 257:208–214. https://doi.org/10.1016/j.bbr.2013.09.030
Bolzoni F, Bruttini C, Esposti R et al (2015) Transcranial direct current stimulation of SMA modulates anticipatory postural adjustments without affecting the primary movement. Behav Brain Res 291:407–413. https://doi.org/10.1016/j.bbr.2015.05.044
Benninger DH, Lomarev M, Lopez G et al (2010) Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:1105–1111. https://doi.org/10.1136/jnnp.2009.202556
Dagan M, Herman T, Harrison R et al (2018) Multitarget transcranial direct current stimulation for freezing of gait in Parkinson’s disease. Mov Disord 33:642–646. https://doi.org/10.1002/mds.27300
Valentino F, Cosentino G, Brighina F et al (2014) Transcranial direct current stimulation for treatment of freezing of gait: a cross-over study. Mov Disord 29:1064–1069. https://doi.org/10.1002/mds.25897
Chang WH, Park E, Cho J-W et al (2017) P029 Effects of dual-mode non-invasive brain stimulation on freezing of gait in patients with Parkinson’s disease. Clin Neurophysiol 128:e23. https://doi.org/10.1016/j.clinph.2016.10.157
Dibble LE, Nicholson DE, Shultz B et al (2004) Sensory cueing effects on maximal speed gait initiation in persons with Parkinson’s disease and healthy elders. Gait Posture 19:215–225. https://doi.org/10.1016/S0966-6362(03)00065-1
Jiang Y, Norman KE (2006) Effects of visual and auditory cues on gait initiation in people with Parkinson’s disease. Clin Rehabil 20:36–45. https://doi.org/10.1191/0269215506cr925oa
Rogers MW, Kennedy R, Palmer S et al (2011) Postural preparation prior to stepping in patients with Parkinson’s disease. J Neurophysiol 106:915–924. https://doi.org/10.1152/jn.00005.2010
Delval A, Moreau C, Bleuse S et al (2014) Auditory cueing of gait initiation in Parkinson’s disease patients with freezing of gait. Clin Neurophysiol. https://doi.org/10.1016/j.clinph.2013.12.101
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Nieuwboer A, Rochester L, Herman T et al (2009) Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture 30:459–463. https://doi.org/10.1016/j.gaitpost.2009.07.108
Schell GR, Strick PL (1984) The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci 4:539–560
Costa-Ribeiro A, Maux A, Bosford T et al (2016) Dopamine-independent effects of combining transcranial direct current stimulation with cued gait training on cortical excitability and functional mobility in Parkinson’s disease. J Rehabil Med 48:819–823. https://doi.org/10.2340/16501977-2134
Lee SY, Kim M-S, Chang WH et al (2014) Effects of repetitive transcranial magnetic stimulation on freezing of gait in patients with Parkinsonism. Restor Neurol Neurosci 32:743–753. https://doi.org/10.3233/RNN-140397
Shirota Y, Ohtsu H, Hamada M et al (2013) Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology 80:1400–1405. https://doi.org/10.1212/WNL.0b013e31828c2f66
Boylan LS, Pullman SL, Lisanby SH et al (2001) Repetitive transcranial magnetic stimulation to SMA worsens complex movements in Parkinson’s disease. Clin Neurophysiol 112:259–264. https://doi.org/10.1016/S1388-2457(00)00519-8
Jeffery DT, Norton JA, Roy FD, Gorassini MA (2007) Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res 182:281–287. https://doi.org/10.1007/s00221-007-1093-y
Madhavan S, Stinear JW (2010) Focal and bidirectional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimul 3:42–50. https://doi.org/10.1016/j.brs.2009.06.005
Broeder S, Nackaerts E, Heremans E et al (2015) Transcranial direct current stimulation in Parkinson’s disease: neurophysiological mechanisms and behavioral effects. Neurosci Biobehav Rev 57:105–117. https://doi.org/10.1016/j.neubiorev.2015.08.010
Ferrucci R, Bocci T, Cortese F et al (2016) Cerebellar transcranial direct current stimulation in neurological disease. Cerebellum Ataxias 3:16. https://doi.org/10.1186/s40673-016-0054-2
Tard C, Dujardin K, Bourriez J-L et al (2014) Attention modulates step initiation postural adjustments in Parkinson freezers. Park Relat Disord 20:284–289. https://doi.org/10.1016/j.parkreldis.2013.11.016
Kaski D, Allum JH, Bronstein AM, Dominguez RO (2014) Applying anodal tDCS during tango dancing in a patient with Parkinson’s disease. Neurosci Lett 568:39–43. https://doi.org/10.1016/j.neulet.2014.03.043
Kaminski E, Steele CJ, Hoff M et al (2016) Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin Neurophysiol 127:2455–2462. https://doi.org/10.1016/j.clinph.2016.03.018
Gallea C, Ewenczyk C, Degos B et al (2017) Pedunculopontine network dysfunction in Parkinson’s disease with postural control and sleep disorders. Mov Disord 32:693–704. https://doi.org/10.1002/mds.26923
Behnke J, Villalba R, Pare J-F et al (2017) Reorganization of thalamocortical glutamatergic synapses in the supplementary motor area (SMA) of MPTP-treated parkinsonian monkeys. In: Program No. 757.08. 2017 neuroscience meeting planner. Society for Neuroscience, Washington, DC
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health [Grant numbers R01 NS070264; R01 NS085188 and P50 NS098573]; the University of Minnesota Neuromodulation Innovations (MnDRIVE) and the National Center for Advancing Translational Sciences of the National Institutes of Health [grant number UL1TR000114]. We are grateful to Bradley Baker, Kyle Ballard, Christina Barth, and Annabel Bavage for their assistance in data post-processing, and our clinical research coordinators, Jacqueline Vachon and Joshua De Kam.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Additional information
Preliminary findings from this study were presented in abstract form at the Society for Neuroscience Meeting in 2015
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, C., Amundsen Huffmaster, S.L., Tuite, P.J. et al. The effects of anodal tDCS over the supplementary motor area on gait initiation in Parkinson’s disease with freezing of gait: a pilot study. J Neurol 265, 2023–2032 (2018). https://doi.org/10.1007/s00415-018-8953-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8953-1