Abstract
Several studies have highlighted the therapeutic potential of transcranial direct current stimulation (tDCS) in patients with neurological diseases, including dementia, epilepsy, post-stroke dysfunctions, movement disorders, and other pathological conditions.
Because of this technique’s ability to modify cerebellar excitability without significant side effects, cerebellar tDCS is a new, interesting, and powerful tool to induce plastic modifications in the cerebellum.
In this report, we review a number of interesting studies on the application of cerebellar tDCS for various neurological conditions (ataxia, Parkinson’s disease, dystonia, essential tremor) and the possible mechanism by which the stimulation acts on the cerebellum.
Study findings indicate that cerebellar tDCS is a promising therapeutic tool in treating several neurological disorders; however, this method’s efficacy appears to be limited, given the current data.
Similar content being viewed by others
Background
The cerebellum is an interesting and fascinating part of the central nervous system, which plays a fundamental role in movement execution and motor control in humans [1].
Beyond its role in balance and motor control, the human cerebellum has been extensively studied for its potential roles in learning, cognition, emotions, and behaviour [2, 3]. Indeed, experiments using neurotropic viruses as transneuronal tracers have demonstrated that the cerebellar output influences not only the primary motor cortex but also the premotor, prefrontal, and parietal areas [3].
The interaction between the cerebellum and the cerebral cortex is based on multiple closed-loop circuits, which have been traditionally considered anatomically and functionally distinct from the cerebro-basal ganglia loops. However, recent anatomical experiments have shown that the dentate nucleus projects disynaptically to the striatum [4] and that the subthalamic nucleus of the basal ganglia projects to the cerebellar cortex [5]. This interconnection between the cerebellum and the basal ganglia provides an explanation for the cerebellar involvement in disorders that are commonly associated with basal ganglia dysfunction (for example, Parkinson’s disease (PD) and dystonia) [6, 7].
Recent findings concerning the anatomical and functional connections between the cerebellum and the basal ganglia may help to characterize the role of the cerebellum in Parkinson’s disease [7]. The cerebellum was shown to receive a dopaminergic projection from the ventral tegmental area/substantia nigra pars compacta [8–10] and to exhibit dopamine D1–3 receptors [11, 12]. Moreover, pathological changes in the cerebellum following dopaminergic degeneration have been reported in patients with Parkinson’s disease and animal models [13, 14]. Functional neuroimaging studies demonstrated increased activation in the cerebellum in patients with PD during motor execution [15–20], during the motor learning process [21–23], as well as in the resting state [24]. A PET study on trial-and-error sequence learning determined that mildly affected patients with PD showed a similar performance with respect to controls but activated four times as much neural tissue, including the cerebellum [25]. It was suggested that the functional role of the increased activity or connectivity in the cerebello-thalamo-cortical loop in PD could be to compensate for hypofunction in the striato-thalamo-cortical circuit [7]. Indeed Yu and colleagues [20] observed a significant negative correlation between the blood oxygen level–dependent activation in the ipsilateral cerebellum and the contralateral putamen while performing a right hand pressing task.
There is strong evidence that the cerebellum also plays an important role in dystonia [6, 26–30]. Recent studies have shown subtle defects in cerebellar Purkinje cells in autopsy specimens from patients with cervical dystonia [31, 32]. Furthermore, imaging studies revealed that abnormalities in the basal ganglia frequently coexist with abnormalities in the cerebellum [33–37]. These data suggest that cerebellum function is incompletely characterized and is probably more expansive than expected.
Because of the multiple cerebellar connections and the broad variety of motor and non-motor functions, the field of cerebellar stimulation with non-invasive techniques (particularly transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS)) is enjoying great success among scientists in the last several years. These techniques enable researchers to investigate neural networks non-invasively and enable them to obtain more information regarding cerebellar physiology and to promote neural plasticity [38].
In 1995, Ugawa [39] found that a single magnetic cerebellar pulse inhibited the amplitude of the motor evoked potential, evoked by TMS delivered a few milliseconds later over the contralateral motor cortex [39, 40]. This inhibition was mediated through the pathway between the cerebellum and the primary motor cortex and was termed cerebellar brain inhibition (CBI).
Controversial results were observed regarding the effects of tDCS on CBI. Galea and colleagues [41] found that anodal tDCS induced an increase of CBI, suggesting that it could act by stimulating tonic Purkinje cell activity, thereby potentiating the inhibitory effect of the dentato-thalamo-cortical pathway on the motor cortex. Nevertheless, Doeltgen and colleagues [42] found that anodal tDCS resulted in a reduction of CBI, suggesting that instead of Purkinje cells, anodal tDCS could modulate superficially located inhibitory interneurons projecting to them, or alternatively, cerebello-thalamo-cortical projections could target inhibitory interneurons within the primary motor cortex. Because of its ability to modify cerebellar excitability without significant side effects, cerebellar tDCS is a new, interesting, and powerful tool to induce plastic modifications in the cerebellum.
In this report, we review a number of interesting studies regarding the application of cerebellar tDCS for different neurological conditions (Table 1) and the possible mechanism by which the stimulation acts on the cerebellum. Cerebellar tDCS has been applied not only to patients affected by primary degenerative cerebellar disorders (e.g., cerebellar ataxias) but also to patients affected by disorders that primarily affect the basal ganglia (e.g., PD, dystonia). Therefore, by modulating cerebellar excitability, cerebellar tDCS could potentially represent a promising therapeutic strategy for movement disorders.
Cerebellar tDCS studies
Evidence from clinical studies suggests that the cerebellum may be involved in the pathophysiology of movement disorders, such as dystonia [6], essential tremor [43], PD [44] and cerebellar ataxia [45], and may be a useful target for tDCS intervention [38, 46].
Parkinson’s disease
Levodopa-induced dyskinesias (LIDs) most likely arise through the cerebello-thalamo-cortical circuit. To evaluate how cerebellar tDCS affects LIDs and cognitive function in PD, Ferrucci and colleagues [47] delivered bilateral anodal (cerebellar tDCS or M1-tDCS) and sham tDCS. After patients received anodal cerebellar tDCS and M1-tDCS for 5 days, the UPDRS IV score (dyskinesias section) improved; conversely, the other variables were unchanged after sham tDCS, cerebellar tDCS, and M1-tDCS.
Despite the small sample size, these preliminary results show that anodal tDCS applied for five consecutive days over the motor cortical areas and the cerebellum improves the LIDs of patients with PD. This finding provides the first evidence that cerebellar tDCS affects LIDs, thus corroborating and extending our current knowledge on the role of the cerebellum in the pathophysiology of PD [7]. Important limitations of this study are the lack of a pharmacological correlation analysis, no inclusion of healthy subjects, and the demonstration that administering L-dopa turns facilitation caused by anodal tDCS into inhibition and prolongs the inhibitory effects of cathodal tDCS.
Dystonia
To investigate the role of the cerebellum in dystonia, Bradnam and colleagues [48] evaluated whether cerebellar tDCS improves handwriting and cyclic drawing kinematics by reducing CBI evoked by TMS in patients with writing dystonia. These researchers examined the kinematic measures, mean stroke frequency with handwriting and fast cyclic drawing, and average pen pressure during light cyclic drawing at baseline in patients and a control group. They examined these measurements after anodal, cathodal, and sham tDCS. The results showed that cerebellar anodal tDCS reduced handwriting mean stroke frequency and average pen pressure, and increased speed and reduced pen pressure during fast cyclic drawing. Kinematic measures were not associated with a decrease in CBI.
Primary dystonia is characterized neurophysiologically by reduced inhibitory mechanisms and an abnormal regulation of plasticity responses. The method commonly used to induce LTP-like (long-term potentiation) plasticity is paired associative stimulation (PAS). This protocol induces plastic changes in excitability in the human motor cortex [49]. In their study, Sadnicka and colleagues [50] used cerebellar tDCS to examine the electrophysiological parameters (resting and active motor threshold, motor cortex excitability, recruitment curves, and cortical silent period) in ten patients with writing dystonia. Patients completed a two-session study (sham and anodal) in which the cerebellum was stimulated along with simultaneous application of PAS.
Patients were also clinically evaluated with the writing movement subscore of the Writer’s Cramp Rating Scale and with a self reported visual analog scale.
The results were negative and do not provide evidence that anodal cerebellar tDCS is beneficial for patients after a single session.
Based on these limited and contradictory results, we conclude that the evidence supporting cerebellar tDCS, as a clinically viable method for treating dystonia is unconvincing. To the best of our knowledge, no one has investigated the effects of consecutive cerebellar tDCS sessions or the effects of combining it with motor training. Further research is needed to investigate ways for increasing the magnitude of the cerebellar tDCS effect.
Essential tremor
Essential tremor (ET) is a common neurological disorder that is characterized by action tremor, which affects the upper limbs in at least 95 % of patients. Tremor is associated with a dysfunction in the basal ganglia and the cerebellar circuit, as well as several neurotransmitter systems projecting to both of these circuits. In a randomized, controlled and crossover study, Gironell and colleagues [51] evaluated the efficacy and safety of ten consecutive cerebellar tDCS sessions in patients with ET. Participants performed a tremor clinical rating scale (TCRS), a test that measures motor performance, daily living activities, or the patients’ subjective assessment, accelerometric recordings and a self-reported disability scale after receiving cathodal stimulation over both cerebellar hemispheres. The authors found no significant differences in the way tDCS affects outcome measures between baseline, day 10 and day 40 (30 days after the last tDCS session).
Although they investigated both the acute and long-lasting effects of tDCS, this small and preliminary study yielded negative results. It is not clear how the four tDCS electrodes were applied over the scalp. The authors have reported two cathodal electrodes placed symmetrically over both cerebellar hemispheres and two anodal electrodes over both prefrontal areas, without specifying if they were unilateral or cross montage. Moreover, it is questionable whether a discomfort questionnaire was administered along with the tDCS procedure and if the subjects were successfully blinded with 2 mA tDCS to the stimulation conditions. It would have been informative if the subjects had been asked if they received real or sham stimulation.
Given the ample evidence in the literature supporting the ability of cerebellar tDCS to enhance motor and non-motor function, the prospect of further trials for neurological disorders is encouraging.
Cerebellar ataxia
Another recent study has investigated the role of tDCS to modulate the activity of the cerebellum in ataxic disorders and has reported interesting results.
To evaluate the effects of anodal tDCS on the cerebellum, Grimaldi and colleagues [52] conducted a study with nine ataxic patients. The authors studied the stretch reflexes (SR) in the upper limbs and examined upper limb dexterity and coordination using a mechanical counter test (MCT). These researchers also used a computerized posturograph during three experimental paradigms. Their results showed that anodal tDCS (1 mA, 20 min) over the right cerebellar hemisphere reduced the amplitude of the long latency SR (LLSR) response, but did not affect the short latency SR response or the MCT score compared to the baseline and sham group. Additionally, the postural parameters remained unchanged following sham or active stimulation of the region in front of the vermis.
These data collectively demonstrated that anodal tDCS applied over the cerebellum reinforces the inhibitory activity exerted by the cerebellar cortex over cerebellar nuclei. tDCS of the cerebellum appears to be a novel method to study the modulation of LLSR by the cerebellar cortex.
In a subsequent study, the same authors [53] performed tDCS over the cerebellum for 20 min immediately followed by tDCS applied over the contralateral motor cortex (20 min, 1 mA) (tCCDCS: transcranial cerebello-cerebral DC stimulation) to assess upper limb tremor and dysmetria in two patients with dominant spinocerebellar ataxia. They found that for the postural tremor, tCCDCS induced a reduction in the amplitude of the oscillations at the level of the index in both patients, as reported by quadratic power spectral density (PSD). tCCDCS also had a positive effect on action tremors, as observed by the drop in the magnitude of low-frequency oscillations from 62.3 to 46.9 % of the baseline values in patient 1 and 2, respectively. Remarkably, following tCCDCS, hypermetria occurred along with a reduction of the onset latency of the antagonist EMG activity in both patients.
Despite the small sample and the lack of follow-up precluding the investigators from assessing how long the effect lasted, these results are highly encouraging. The combination of tDCS of the cerebellum with tDCS of the motor/premotor cortex demonstrated that this technique may be considered a symptomatic therapeutic strategy for upper limb deficits in disabling cerebellar ataxia, representing a novel approach in the field of cerebellar neuromodulation.
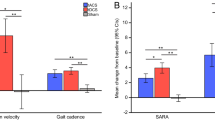
An interesting therapeutic effect was also observed by Benussi and colleagues [54]. In a double-blind, randomized, sham-controlled study, they investigated whether a single session of cerebellar anodal transcranial direct current stimulation could improve symptoms in nine patients with ataxia. Each patient received both anodal and sham cerebellar tDCS stimulation (20 min, 2 mA) in two different sessions, separated by at least 1 week.
After cerebellar tDCS, there was a significant improvement in the functional clinical scores, as observed with the Scale for the Assessment and Rating of Ataxia (SARA), the International Cooperative Ataxia Rating Scale (ICARS), and in motor task measurement with the nine-hole peg test (9HPT) and 8-Meter Walking Time (8 MW) assessment within the entire cohort of patients. Specifically, the individual assessments of the four subscores on the ICARS scale revealed a significant improvement in the posture, gait and limb coordination subscores. Moreover, single-group analysis in the SCA (spinocerebellar ataxia) and the cerebellar variant of MSA (multiple system atrophy, MSA-C) cohorts demonstrated a significant effect from anodal cerebellar tDCS on SARA, ICARS, and 9HPT testing. Only in the SCA group was there a significant difference in the 8MW testing. There was no significant difference in the MSA-C group.
The investigators concluded that a single stimulation session applied to the cerebellar cortex can temporarily improve symptoms in patients with ataxia and might have therapeutic potential in these patients, but more powerful stimulation may be needed.
These preliminarily studies show that cerebellar tDCS is safe and can improve neurological symptoms, especially in patients with ataxia. While this finding is promising, the clinical efficacy of this technique in patients with neurological disorders remains to be definitively established by large, controlled clinical studies. Future research efforts should be directed toward identifying the optimal stimulation parameters (electrode montage and size, duration, intensity, number of sessions, on-line vs. off-line, duration of treatment), and potentially targeting specific types of neurological disorders or individual patients.
Mechanisms of action
Two main questions concerning the mechanism of action of cerebellar tDCS are still unanswered: 1) whether cerebellar tDCS has polarity-specific effects or not and 2) whether it modulates cerebellar activity during or following a behavioural intervention (on-line versus off-line effects).
Differences in the reported effects are mainly due to the function explored, as different functions rely on different cerebellar areas with variable neural substrates and axonal orientation to the electrical field. Some authors have reported polarity-specific effects [41, 55–57], while others have not [58, 59]. This apparent discrepancy may be due to differences in the electrode size and the montage, as well as variations in the stimulation intensity [60]. In particular, while studies exploring cognitive and emotional domains have used a classical monopolar configuration, others focusing on motor functions have adopted a different montage, in which the return electrode is placed over the ipsilateral face. Only in the last study montage have polarity-specific effects been demonstrated [41].
Cerebellar tDCS probably has both on-line and off-line effects on cerebellar excitability. This finding would be in concordance with the effects elicited by tDCS in the cerebral cortex that are observable after both short-term and long-term delays and most likely interfering with long-term potentiation (LTP-like) phenomena [61]. From a cellular point of view, animal studies seem to suggest that the electrical stimulation of Purkinje cells mediates on-line effects [62], whereas depolarization of Golgi inhibitory neurons is responsible for long-lasting changes [63]. Purkinje cells represent the sole output from the cerebellar cortex, and their activation leads to the inhibition of the dentate nucleus, ultimately dampening motor cortex excitability, a phenomenon referred to as CBI [40]. Cerebellar tDCS may interfere with this connectivity, depending on its polarity and intensity. Nonetheless, electrical fields induced by cerebellar tDCS in humans are much smaller than those used in animals, thus making it difficult to compare their mechanisms of action [64, 65].
From a molecular perspective, cerebellar polarization may act on different neurotransmitters and signalling pathways [66]. For instance, the two main neurotransmitters in the cerebellum (glutamate and γ-amino-butyric acid) are both modulated when tDCS is applied over the sensorimotor areas [66]. In addition, the concentration of cerebellar myo-inositol is likely modified by weak electrical fields, as is the case for transcranial current stimulation [67]. Finally, cerebellar tDCS may interfere with dopaminergic transmission, probably through disynaptic pathways leading from the cerebellum to the intralaminar nuclei of the thalamus and to the dorsolateral putamen [4]. The cerebellum itself expresses all types of dopamine receptors that share similar properties with the striatal dopaminergic system [68]. The cerebellum also down-regulates the striatal D1 receptors through the deep nuclei and the thalamic relay [11].
As a whole, anodal and cathodal cerebellar tDCS likely exert effects through different, rather than simply specular, mechanisms of action on different cellular and molecular targets, in concordance with those reported both for the cerebral cortex [66] and spinal cord [56]. The polarity of cerebellar tDCS after-effects may also depend on the pre-existing excitability state, as well as with task-induced activity, as described for cortical stimulation [69]. Elucidation of the physiological variables underlying tDCS after-effects and evidence for the existence of a cerebellar homeostatic-like plasticity would be critical areas to investigate extensively in future studies.
Conclusions
Cerebellar tDCS is a novel, safe and effective neurostimulation technique for non-invasive modulation of cerebellar excitability. Depending of the different pathophysiologies of human diseases, it has been successfully used for the treatment of movement disorders, ranging from cerebellar ataxia to dystonia, as well as for the control of levodopa-induced dyskinesias in Parkinson’s disease patients. Modelling studies have demonstrated that the cerebellum is the structure mainly affected by cerebellar tDCS, without a significant current spreading to the brainstem or the occipital cortex.
Studies on healthy subjects have demonstrated that cerebellar tDCS influences the pain experience, nociceptive perception [55, 56], and cognitive function [46].
However, further studies are needed to elucidate this technique’s mechanisms of action and areas of influence. Moreover, it is worth remembering that cerebellar involvement in non-motor (i.e., cognitive) functions remains to be systematically evaluated.
Despite the wide heterogeneity of the limited data available for review, we will try to offer some practical operative suggestions for those wishing to approach cerebellar tDCS to treat patients with neurological disease.
First, whereas anodal tDCS improves motor functions over the right cerebellum, cathodal cerebellar tDCS seems to be ineffective. The second suggestion concerns stimulation duration and intensity. The optimal repetition rate and duration to promote tDCS-induced plasticity remains undetermined.
A reasonable choice might be 2 mA for 20 min, using a common electrode size of 35 cm2 (generating charge densities ranging from 0.034 to 0.068 C/cm2) in repeated daily sessions (5 days). For instance, given that a recent study of major depression concluded that treatment should continue for several weeks or should combine stimulation of the cerebellum and cerebral cortex to achieve an optimal clinical response, the same might apply to neurologic patients. At present, because most of the available data on cerebellar tDCS-induced motor improvement concerns patients with ataxia, these patients appear to be the most likely to respond positively.
Further research efforts should aim to identify the optimal stimulation parameters (site, electrode montage and size, duration, intensity, number of sessions, online vs. offline, duration of treatment), potentially targeting specific types of neurological disease and individual patients.
Although current findings indicate that cerebellar tDCS may be a promising therapeutic tool for treating several neurological disorders, its efficacy appears limited and awaits further substantiation in large, randomised controlled clinical studies.
References
Groiss SJ, Ugawa Y. Cerebellum. Handb Clin Neurol. 2013;116:643–53.
Reeber SL, Otis TS, Sillitoe RV. New roles for the cerebellum in health and disease. Front Syst Neurosci. 2013;7:83.
Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34.
Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8(11):1491–3.
Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107(18):8452–6.
Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35.
Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136(Pt 3):696–709.
Ikai Y, Takada M, Shinonaga Y, Mizuno N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 1992;51(3):719–28.
Melchitzky DS, Lewis DA. Tyrosine hydroxylase- and dopamine transporter-immunoreactive axons in the primate cerebellum. Evidence for a lobular- and laminar-specific dopamine innervation. Neuropsychopharmacology. 2000;22(5):466–72.
Panagopoulos NT, Papadopoulos GC, Matsokis NA. Dopaminergic innervation and binding in the rat cerebellum. Neurosci Lett. 1991;130(2):208–12.
Giompres P, Delis F. Dopamine transporters in the cerebellum of mutant mice. Cerebellum (London, England). 2005;4(2):105–11.
Hurley MJ, Mash DC, Jenner P. Markers for dopaminergic neurotransmission in the cerebellum in normal individuals and patients with Parkinson’s disease examined by RT-PCR. Eur J Neurosci. 2003;18(9):2668–72.
Heman P, Barcia C, Gomez A, Ros CM, Ros-Bernal F, Yuste JE, et al. Nigral degeneration correlates with persistent activation of cerebellar Purkinje cells in MPTP-treated monkeys. Histol Histopathol. 2012;27(1):89–94.
Rolland AS, Herrero MT, Garcia-Martinez V, Ruberg M, Hirsch EC, Francois C. Metabolic activity of cerebellar and basal ganglia-thalamic neurons is reduced in parkinsonism. Brain. 2007;130(Pt 1):265–75.
Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain. 1999;122(Pt 3):483–95.
Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, et al. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997;120(Pt 1):103–10.
Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128(Pt 10):2250–9.
Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79(7):760–6.
Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain. 2010;133(Pt 8):2394–409.
Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. NeuroImage. 2007;35(1):222–33.
Bedard P, Sanes JN. On a basal ganglia role in learning and rehearsing visual-motor associations. NeuroImage. 2009;47(4):1701–10.
Mallol R, Barros-Loscertales A, Lopez M, Belloch V, Parcet MA, Avila C. Compensatory cortical mechanisms in Parkinson’s disease evidenced with fMRI during the performance of pre-learned sequential movements. Brain Res. 2007;1147:265–71.
Werheid K, Zysset S, Muller A, Reuter M, von Cramon DY. Rule learning in a serial reaction time task: an fMRI study on patients with early Parkinson’s disease. Brain Res. 2003;16(2):273–84.
Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, et al. Regional homogeneity changes in patients with Parkinson’s disease. Hum Brain Mapp. 2009;30(5):1502–10.
Mentis MJ, Dhawan V, Nakamura T, Ghilardi MF, Feigin A, Edwards C, et al. Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology. 2003;60(4):612–9.
Avanzino L, Abbruzzese G. How does the cerebellum contribute to the pathophysiology of dystonia. Basal Ganglia. 2012;2:231–5.
Filip P, Lungu OV, Bares M. Dystonia and the cerebellum: a new field of interest in movement disorders? Clin Neurophysiol. 2013;124(7):1269–76.
Lehericy S, Tijssen MA, Vidailhet M, Kaji R, Meunier S. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord. 2013;28(7):944–57.
Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42(2):185–201.
Sadnicka A, Hoffland BS, Bhatia KP, van de Warrenburg BP, Edwards MJ. The cerebellum in dystonia - help or hindrance? Clin Neurophysiol. 2012;123(1):65–70.
Ma K, Babij R, Cortes E, Vonsattel JP, Louis ED. Cerebellar pathology of a dual clinical diagnosis: patients with essential tremor and dystonia. Tremor Other Hyperkinet Mov (NY). 2012;2. doi:10.7916/D8JD4VJ5.
Prudente CN, Pardo CA, Xiao J, Hanfelt J, Hess EJ, Ledoux MS, et al. Neuropathology of cervical dystonia. Exp Neurol. 2012;241:95–104.
Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29(31):9740–7.
Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69(4):376–80.
Hutchinson M, Nakamura T, Moeller JR, Antonini A, Belakhlef A, Dhawan V, et al. The metabolic topography of essential blepharospasm: a focal dystonia with general implications. Neurology. 2000;55(5):673–7.
Odergren T, Stone-Elander S, Ingvar M. Cerebral and cerebellar activation in correlation to the action-induced dystonia in writer’s cramp. Mov Disord. 1998;13(3):497–508.
Thobois S, Ballanger B, Xie-Brustolin J, Damier P, Durif F, Azulay JP, et al. Globus pallidus stimulation reduces frontal hyperactivity in tardive dystonia. J Cereb Blood Flow Metab. 2008;28(6):1127–38.
Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, et al. Non-invasive cerebellar stimulation--a consensus paper. Cerebellum (London, England). 2014;13(1):121–38.
Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37(6):703–13.
Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol. 1991;441:57–72.
Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29(28):9115–22.
Doeltgen SH, Young J, Bradnam LV. Anodal direct current stimulation of the cerebellum reduces cerebellar brain inhibition but does not influence afferent input from the hand or face in healthy adults. Cerebellum (London, England). 2016;15(4):466–74.
Louis ED. Essential tremor: from bedside to bench and back to bedside. Curr Opin Neurol. 2014;27(4):461–7.
Lewis MM, Galley S, Johnson S, Stevenson J, Huang X, McKeown MJ. The role of the cerebellum in the pathophysiology of Parkinson’s disease. Can J Neurol Sci. 2013;40(3):299–306.
Tada M, Nishizawa M, Onodera O. Redefining cerebellar ataxia in degenerative ataxias: lessons from recent research on cerebellar systems. J Neurol Neurosurg Psychiatry. 2015;86(8):922–8.
Ferrucci R, Priori A. Transcranial cerebellar direct current stimulation (tcDCS): motor control, cognition, learning and emotions. NeuroImage. 2014;85(Pt 3):918–23.
Ferrucci R, Cortese F, Bianchi M, Pittera D, Turrone R, Bocci T, et al. Cerebellar and motor cortical transcranial stimulation decrease Levodopa-induced Dyskinesias in Parkinson’s disease. Cerebellum (London, England). 2015;15(1):43–7.
Bradnam LV, Graetz LJ, McDonnell MN, Ridding MC. Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front Hum Neurosci. 2015;9:286.
Classen J, Wolters A, Stefan K, Wycislo M, Sandbrink F, Schmidt A, et al. Paired associative stimulation. Suppl Clin Neurophysiol. 2004;57:563–9.
Sadnicka A, Hamada M, Bhatia KP, Rothwell JC, Edwards MJ. Cerebellar stimulation fails to modulate motor cortex plasticity in writing dystonia. Mov Disord. 2014;29(10):1304–7.
Gironell A, Martinez-Horta S, Aguilar S, Torres V, Pagonabarraga J, Pascual-Sedano B, et al. Transcranial direct current stimulation of the cerebellum in essential tremor: a controlled study. Brain Stimul. 2014;7(3):491–2.
Grimaldi G, Manto M. Anodal transcranial direct current stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann Biomed Eng. 2013;41(11):2437–47.
Grimaldi G, Oulad Ben Taib N, Manto M, Bodranghien F. Marked reduction of cerebellar deficits in upper limbs following transcranial cerebello-cerebral DC stimulation: tremor reduction and re-programming of the timing of antagonist commands. Front Syst Neurosci. 2014;8:9.
Benussi A, Koch G, Cotelli M, Padovani A, Borroni B. Cerebellar transcranial direct current stimulation in patients with ataxia: a double-blind, randomized, sham-controlled study. Mov Disord. 2015;30(12):1701–5.
Bocci T, Barloscio D, Parenti L, Sartucci F, Carli G, Santarcangelo EL. High Hypnotizability Impairs the Cerebellar Control of Pain. Cerebellum (London, England). 2016; doi:10.1007/s12311-016-0764-2.
Bocci T, Santarcangelo E, Vannini B, Torzini A, Carli G, Ferrucci R, et al. Cerebellar direct current stimulation modulates pain perception in humans. Restor Neurol Neurosci. 2015;33(5):597–609.
Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012;107(11):2950–7.
Ferrucci R, Giannicola G, Rosa M, Fumagalli M, Boggio PS, Hallett M, et al. Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cognit Emot. 2009;26(5):786–99.
Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, et al. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci. 2008;20(9):1687–97.
Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591(7):1987–2000.
Hamada M, Strigaro G, Murase N, Sadnicka A, Galea JM, Edwards MJ, et al. Cerebellar modulation of human associative plasticity. J Physiol. 2012;590(10):2365–74.
Chan CY, Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol. 1986;371:89–114.
Hull CA, Chu Y, Thanawala M, Regehr WG. Hyperpolarization induces a long-term increase in the spontaneous firing rate of cerebellar Golgi cells. J Neurosci. 2013;33(14):5895–902.
Parazzini M, Rossi E, Ferrucci R, Fiocchi S, Liorni I, Priori A, et al. Computational model of cerebellar transcranial direct current stimulation. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:237–40.
Parazzini M, Rossi E, Ferrucci R, Liorni I, Priori A, Ravazzani P. Modelling the electric field and the current density generated by cerebellar transcranial DC stimulation in humans. Clin Neurophysiol. 2014;125(3):577–84.
Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29(16):5202–6.
Rango M, Cogiamanian F, Marceglia S, Barberis B, Arighi A, Biondetti P, et al. Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: a 1H-MRS study. Magn Reson Med. 2008;60(4):782–9.
Kishore A, Popa T. Cerebellum in levodopa-induced dyskinesias: the unusual suspect in the motor network. Front Neurol. 2014;5:157.
Bortoletto M, Pellicciari MC, Rodella C, Miniussi C. The interaction with task-induced activity is more important than polarization: a tDCS study. Brain Stimul. 2015;8(2):269–76.
Acknowledgment
Department of Health Sciences of the University of Milan has been partly supported by the Aldo Ravelli Donation for research on Parkinson's disease.
Availability of data and materials
Not applicable because it is a review of already published literature.
Authors’ contributions
RF, TB, FC, FR, AP conceptualized and drafted this review article. All of the co-authors critically revised the manuscript for intellectual content and read and approved the final manuscript.
Competing interests
Roberta Ferrucci and Alberto Priori are stakeholders in Newronika s.r.l., a spin-off company of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and the Università degli Studi di Milano.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Grant/Financial Support
The authors declare that they have no financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ferrucci, R., Bocci, T., Cortese, F. et al. Cerebellar transcranial direct current stimulation in neurological disease. cerebellum ataxias 3, 16 (2016). https://doi.org/10.1186/s40673-016-0054-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40673-016-0054-2