Abstract
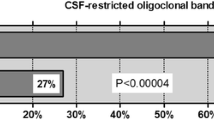
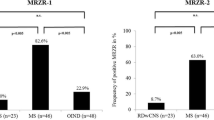
Some patients with primary central nervous system lymphoma (PCNSL) may initially present with similar clinical, magnetic resonance imaging, and routine cerebrospinal fluid (CSF) findings as those observed in multiple sclerosis (MS). The MRZ reaction (MRZR), composed of the three respective antibody indices (AIs) against measles, rubella, and varicella zoster virus, appears to be the most specific CSF marker for MS. This study aimed to determine whether a positive MRZR and other routine CSF markers help differentiate between MS and PCNSL. Data regarding brain biopsy, CSF routine tests, cytopathological examination and immunophenotyping of CSF cells were assessed in 68 PCNSL patients. MRZR was determined, as possible, in PCNSL patients (n = 37) and in those with MS (n = 74; age and sex matched to PSCNL patients) and psychiatric disorders (PD; n = 78). Two stringency levels for a positive antibody index (AI) evaluation (AI ≥ 1.5 and 2.0) were applied, and MRZR was considered positive in cases with ≥ 2 positive AIs (MRZR-2). Using the common AI threshold of ≥ 1.5, MS patients exhibited positive MRZR-2 (58.1%) more frequently than PCNSL (8.1%) and PD patients (2.6%; p < 0.0001 for each comparison with the MS group) corresponding to a positive predictive value (PPV) of 89.6% and a negative predictive value (NPV) of 78.0%. On applying the stricter AI threshold of ≥ 2.0, 37.8% of MS patients were MRZR-2 positive; however, all patients with PCNSL and PD were MRZR-2 negative (p < 0.0001 for each comparison with the MS cohort) resulting in a PPV of 100% and an NPV of 71.4%. Consequently, a positive MRZR-2 result may contribute toward the distinction between MS and PCNSL owing to its high specificity and PPV for MS in the context of the present study. Among the other CSF parameters only a quantitative intrathecal IgG synthesis (present in 49.3% of MS patients but in none of the PCNSL or PD patients; p < 0.0001 for each comparison with the MS group) reliably indicated MS rather than PCNSL.

Similar content being viewed by others
Abbreviations
- AI:
-
Antibody index
- AIs:
-
Antibody indices
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- ECOG PS:
-
Eastern Cooperative Oncology Group Performance Status
- Ig:
-
Immunoglobulin
- IgG/M/A:
-
Immunoglobulin G/M/A
- LDH:
-
Lactate dehydrogenase
- LP:
-
Lumbar puncture
- MRI:
-
Magnetic resonance imaging
- MRZR:
-
Measles virus, rubella virus, and varicella zoster virus reaction
- MS:
-
Multiple sclerosis
- NPV:
-
Negative predictive value
- OCB:
-
Oligoclonal bands
- PCNSL:
-
Primary central nervous system lymphoma
- PD:
-
Psychiatric disorders
- PPMS:
-
Primary progressive MS
- PPV:
-
Positive predictive value
- QAlb:
-
Albumin quotient
- RRMS:
-
Relapsing–remitting MS
- SD:
-
Standard deviation
References
Grommes C, DeAngelis LM (2017) Primary CNS lymphoma. J Clin Oncol JCO2017727602. https://doi.org/10.1200/jco.2017.72.7602
Ostrom QT, Gittleman H, Xu J et al (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neurooncology 18(suppl_5):v1–v75. https://doi.org/10.1093/neuonc/now207
Bataille B, Delwail V, Menet E et al (2000) Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg 92(2):261–266. https://doi.org/10.3171/jns.2000.92.2.0261
Schabet M, Herrlinger U, Weller M et al (1997) Neue Entwicklungen in Diagnostik und Therapie primärer non-Hodgkin-Lymphome des zentralen Nervensystems (New developments in diagnosis and therapy of primary non-Hodgkin’s lymphoma of the central nervous system). Nervenarzt 68(4):298–308
Grimm SA, Pulido JS, Jahnke K et al (2007) Primary intraocular lymphoma: an international primary central nervous system lymphoma collaborative group report. Ann Oncol 18(11):1851–1855. https://doi.org/10.1093/annonc/mdm340
Hottenrott T, Dersch R, Berger B et al (2017) The MRZ reaction in primary progressive multiple sclerosis. Fluids Barriers CNS 14(1):2. https://doi.org/10.1186/s12987-016-0049-7
Fischer L, Martus P, Weller M et al (2008) Meningeal dissemination in primary CNS lymphoma: prospective evaluation of 282 patients. Neurology 71(14):1102–1108. https://doi.org/10.1212/01.wnl.0000326958.52546.f5
Korfel A, Weller M, Martus P et al (2012) Prognostic impact of meningeal dissemination in primary CNS lymphoma (PCNSL): experience from the G-PCNSL-SG1 trial. Ann Oncol 23(9):2374–2380. https://doi.org/10.1093/annonc/mdr627
Abrey LE, Batchelor TT, Ferreri AJM et al (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23(22):5034–5043. https://doi.org/10.1200/jco.2005.13.524
Felgenhauer K, Reiber H (1992) The diagnostic significance of antibody specificity indices in multiple sclerosis and herpes virus induced diseases of the nervous system. Clin Investig 70(1):28–37
Jarius S, Eichhorn P, Franciotta D et al (2017) The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature. J Neurol 264(3):453–466. https://doi.org/10.1007/s00415-016-8360-4
Wurster U, Stachan R, Windhagen A et al (2006) Reference values for standard cerebrospinal fluid examinations in multiple sclerosis. Results from 99 healthy volunteers. Mult Scler 12:P248
Jarius S, Franciotta D, Bergamaschi R et al (2008) Polyspecific, antiviral immune response distinguishes multiple sclerosis and neuromyelitis optica. J Neurol Neurosurg Psychiatry 79(10):1134–1136. https://doi.org/10.1136/jnnp.2007.133330
Jarius S, Eichhorn P, Jacobi C et al (2009) The intrathecal, polyspecific antiviral immune response: specific for MS or a general marker of CNS autoimmunity? J Neurol Sci 280(1–2):98–100. https://doi.org/10.1016/j.jns.2008.08.002
Hottenrott T, Dersch R, Berger B et al (2015) The intrathecal, polyspecific antiviral immune response in neurosarcoidosis, acute disseminated encephalomyelitis and autoimmune encephalitis compared to multiple sclerosis in a tertiary hospital cohort. Fluids Barriers CNS 12:27. https://doi.org/10.1186/s12987-015-0024-8
Bednárová J, Stourac P, Adam P (2005) Relevance of immunological variables in neuroborreliosis and multiple sclerosis. Acta Neurol Scand 112(2):97–102. https://doi.org/10.1111/j.1600-0404.2005.00418.x
Puccioni-Sohler M, Kitze B, Felgenhauer K et al (1995) The value of CSF analysis for the differential diagnosis of HTLV-I associated myelopathy and multiple sclerosis. Arq Neuropsiquiatr 53(4):760–765
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Kaller CP, Loosli SV, Rahm B et al (2014) Working memory in schizophrenia: behavioral and neural evidence for reduced susceptibility to item-specific proactive interference. Biol Psychiatry 76(6):486–494. https://doi.org/10.1016/j.biopsych.2014.03.012
Endres D, Huzly D, Dersch R, Stich O, Berger B, Schuchardt F, Perlov E, Venhoff N, Hellwig S, Fiebich BL, Erny D, Hottenrott T, Tebartz van Elst L (2017) Do patients with schizophreniform and bipolar disorders show an intrathecal, polyspecific, antiviral immune response? A pilot study. Fluids Barriers CNS 14(1):34. https://doi.org/10.1186/s12987-017-0082-1
Reiber H (1998) Cerebrospinal fluid-physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult Scler 4(3):99–107. https://doi.org/10.1177/135245859800400302
Reiber H, Ungefehr S, Jacobi C (1998) The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult Scler 4(3):111–117. https://doi.org/10.1177/135245859800400304
Derfuss T, Hohlfeld R, Meinl E (2001) Multiple sclerosis. Chlamydia hypothesis in debate (Multiple Sklerose. Chlamydien-Hypothese in der Diskussion). Nervenarzt 72(10):820–823
Reiber H (1994) Flow rate of cerebrospinal fluid (CSF)—a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci 122(2):189–203
Graef IT, Henze T, Reiber H (1994) Polyspezifische Immunreaktion im ZNS bei Autoimmunerkrankungen mit ZNS-Beteiligung (Polyspecific immune reaction in the central nervous system in autoimmune diseases with CNS involvement). Z Arztl Fortbild (Jena) 88(7–8):587–591
Reiber H (2016) Cerebrospinal fluid data compilation and knowledge-based interpretation of bacterial, viral, parasitic, oncological, chronic inflammatory and demyelinating diseases. Diagnostic patterns not to be missed in neurology and psychiatry. Arq Neuropsiquiatr 74(4):337–350. https://doi.org/10.1590/0004-282x20160044
Rosche B, Laurent S, Conradi S et al (2012) Measles IgG antibody index correlates with T2 lesion load on MRI in patients with early multiple sclerosis. PLoS One 7(1):e28094. https://doi.org/10.1371/journal.pone.0028094
Kułakowska A, Mroczko B, Mantur M et al (2012) Multiplexing analysis of the polyspecific intrathecal immune response in multiple sclerosis. Methods 56(4):528–531. https://doi.org/10.1016/j.ymeth.2012.03.002
Reiber H, Peter JB (2001) Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 184(2):101–122
Liebsch R, Kornhuber ME, Dietl D et al (1996) Blood-CSF barrier integrity in multiple sclerosis. Acta Neurol Scand 94(6):404–410
Baraniskin A, Kuhnhenn J, Schlegel U et al (2011) Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 117(11):3140–3146. https://doi.org/10.1182/blood-2010-09-308684
Ohe Y, Hayashi T, Mishima K et al (2013) Central nervous system lymphoma initially diagnosed as tumefactive multiple sclerosis after brain biopsy. Intern Med 52(4):483–488
Kuroda Y, Kawasaki T, Haraoka S et al (1992) Autopsy report of primary CNS B-cell lymphoma indistinguishable from multiple sclerosis: diagnosis with the immunoglobulin gene rearrangements analysis. J Neurol Sci 111(2):173–179
Chayasirisobhon S, Kumar V, Ali I et al (1987) Primary lymphoma of the central nervous system: a diagnostic problem. J Natl Med Assoc 79(2):198–200
Bessell EM, Dickinson P, Dickinson S et al (2011) Increasing age at diagnosis and worsening renal function in patients with primary central nervous system lymphoma. J Neurooncol 104(1):191–193. https://doi.org/10.1007/s11060-010-0457-5
Reiber H, Teut M, Pohl D et al (2009) Paediatric and adult multiple sclerosis: age-related differences and time course of the neuroimmunological response in cerebrospinal fluid. Mult Scler 15(12):1466–1480. https://doi.org/10.1177/1352458509348418
Robinson-Agramonte M, Reiber H, Cabrera-Gomez JA et al (2007) Intrathecal polyspecific immune response to neurotropic viruses in multiple sclerosis: a comparative report from Cuban patients. Acta Neurol Scand 115(5):312–318. https://doi.org/10.1111/j.1600-0404.2006.00755.x
Tumani H, Deisenhammer F, Giovannoni G et al (2011) Revised McDonald criteria: The persisting importance of cerebrospinal fluid analysis. Ann Neurol 70(3):520. https://doi.org/10.1002/ana.22508 (author reply 521)
Acknowledgements
Special thanks are due to the staff of the Institute of Virology who performed the immunoassays for the MRZ-AI and to the staff of the CSF laboratory at the Department of Neurology who analysed the CSF/serum samples for the routine parameters.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The cost of the article-processing charge was covered by the Albert Ludwig University of Freiburg.
Author information
Authors and Affiliations
Contributions
TH and ES initiated this study. TH performed parts of the statistical analyses and wrote the manuscript. ES and KF identified the PCNSL patients; DE and LTvE provided the psychiatric patients group. RD performed parts of the statistical analyses. DH supervised performance of the immunoassays in the Institute of Virology. OS supervised the performance of the CSF analyses. OS and DE supervised the study. All authors helped to draft the manuscript, read and approved the final manuscript version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee of the University Freiburg approved the study (EK-Fr 489/14).
Consent for publication
Not applicable.
Availability of data and supporting materials section
The data that support the findings of this study are included in this study or available from the corresponding author upon reasonable request.
Conflicts of interest
TH received travel grants from Bayer Vital GmbH and Novartis. ES, KF, RD and DE report no conflicts of interest with this study. BB received travel grants and/or training expenses from Bayer Vital GmbH, Ipsen Pharma GmbH, Norvartis, and Genzyme, as well as lecture fees from Ipsen Pharma GmbH. DH received lecture fees from Serion. LTvE received consulting and lecture fees, grant and research support from Eli Lilly, Janssen-Cilag, Novartis, Shire, UCB, GSK, Servier, Janssen, and Cyberonics. OS and SR report receiving consulting and lecture fees, grant and research support from Baxter, Bayer Vital GmbH, Biogen Idec, Genzyme, Merck Serono, Novartis, RG, Sanofi-Aventis and Teva. Furthermore, SR indicates that he is a founding executive board member of ravo Diagnostika GmbH (Oltmannsstraße 5, D-79100 Freiburg, Germany), which is selling in vitro diagnostic medical devices for the detection of infectious diseases and paraneoplastic autoantibodies.
Author information
All authors except OS are doctors working at the Medical Centre-University Freiburg, Germany; OS is working as a consultant physician at the medical care unit in Konstanz, Germany. TH, RD and DE are resident physicians; ES and KF are consultant physicians; BB and DH are senior physicians. SR and LTvE are chief physicians.
Rights and permissions
About this article
Cite this article
Hottenrott, T., Schorb, E., Fritsch, K. et al. The MRZ reaction and a quantitative intrathecal IgG synthesis may be helpful to differentiate between primary central nervous system lymphoma and multiple sclerosis. J Neurol 265, 1106–1114 (2018). https://doi.org/10.1007/s00415-018-8779-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8779-x