Abstract
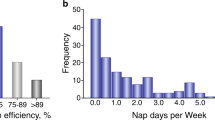
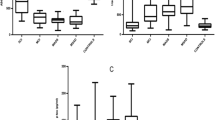
Hypothalamus is a key brain region regulating several essential homeostatic functions, including the sleep–wake cycle. Alzheimer’s disease (AD) pathology affects nuclei controlling sleep–wake rhythm sited in this brain area. Since only post-mortem studies documented the relationship between hypothalamic atrophy and sleep–wake cycle impairment, we investigated in AD patients the possible hypothalamic in vivo alteration using 2-deoxy-2-(18F) fluoro-d-glucose ([18F]FDG) positron emission tomography ([18F]FDG PET), and its correlations with sleep impairment and cerebrospinal-fluid (CSF) AD biomarkers (tau proteins and β-amyloid42). We measured sleep by polysomnography, CSF AD biomarkers and orexin levels, and hypothalamic [18F]FDG PET uptake in a population of AD patients compared to age- and sex-matched controls. We documented the significant reduction of hypothalamic [18F]FDG PET uptake in AD patients (n = 18) compared to controls (n = 18) (p < 0.01). Moreover, we found the increase of CSF orexin levels coupled with the marked alteration of nocturnal sleep in AD patients than controls. We observed the significant association linking the reduction of both sleep efficiency and REM sleep to the reduction of hypothalamic [18F]FDG PET uptake in the AD group, which in turn negatively correlated with the total-tau/beta-amyloid42 ratio (index of more marked neurodegeneration). Moreover, controls but not AD patients showed [18F]FDG PET interconnections between hypothalamus and limbic system. We documented the in vivo dysfunction of hypothalamus in AD patients, which lost the physiological connections with limbic system and was correlated with both nocturnal sleep disruption and CSF AD biomarkers.


Similar content being viewed by others
References
Saper CB, Lowell BB (2014) The hypothalamus. Curr Biol 24(23):R1111–R1116
Baloyannis SJ, Mavroudis I, Mitilineos D, Baloyannis IS, Costa VG (2015) The hypothalamus in Alzheimer’s disease: a Golgi and electron microscope study. Am J Alzheimers Dis Other Demen 30(5):478–487
Ishii M, Iadecola C (2015) Metabolic and non-cognitive manifestations of Alzheimer’s disease: the hypothalamus as both culprit and target of pathology. Cell Metab 22(5):761–776
Shan L, Bossers K, Unmehopa U, Bao AM, Swaab DF (2012) Alterations in the histaminergic system in Alzheimer’s disease: a postmortem study. Neurobiol Aging 33(11):2585–2598
Fronczek R, van Geest S, Frölich M, Overeem S, Roelandse FW, Lammers GJ, Swaab DF (2012) Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging 33(8):1642–1650
Yaari R, Corey-Bloom J (2007) Alzheimer’s disease. Semin Neurol 27(1):32–41
Vitiello MV, Prinz PN (1989) Alzheimer’s disease. Sleep and sleep/wake patterns. Clin Geriatr Med 5(2):289–299
Liguori C, Romigi A, Nuccetelli M et al (2014) Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol 71(12):1498–1505
Beaulieu-Bonneau S, Hudon C (2009) Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr 21(4):654–666
Bonanni E, Maestri M, Tognoni G et al (2005) Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. J Sleep Res 14(3):311–317
Montplaisir J, Petit D, Lorrain D et al (1995) Sleep in Alzheimer’s disease: further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. Sleep 18(3):145–148
Maestri M, Carnicelli L, Tognoni G et al (2015) Non-rapid eye movement sleep instability in mild cognitive impairment: a pilot study. Sleep Med 16(9):1139–1145
Power AE (2004) Slow-wave sleep, acetylcholine, and memory consolidation. Proc Natl Acad Sci USA 101:1795–1796
Wang JL, Lim AS, Chiang WY et al (2015) Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol 78(2):317–322
Lim AS, Ellison BA, Wang JL et al (2014) Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain 137(Pt 10):2847–2861
Liguori C, Nuccetelli M, Izzi F et al (2016) Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer’s disease. Neurobiol Aging 40:120–126
Varrone A, Asenbaum S, Vander Borght T et al (2009) European Association of Nuclear Medicine Neuroimaging Committee. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging 36(12):2103–2110
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):263–269
Romigi A, Liguori C, Placidi F et al (2014) Sleep disorders in spinal and bulbar muscular atrophy (Kennedy’s disease): a controlled polysomnographic and self-reported questionnaires study. J Neurol 261(5):889–893
Iber C, Ancoli-Israel S, Chesson A, Quan S, for the American Academy of Sleep Medicine (2007) The AASM manual for the scoring of sleep and associated events; rules, terminology and technical specifications, 1st edn. American Academy of Sleep Medicine, Westchester
Liguori C, Olivola E, Pierantozzi M et al (2016) Cerebrospinal-fluid Alzheimer’s disease biomarkers and blood-brain barrier integrity in a natural population of cognitive intact Parkinson’s disease patients. CNS Neurol Disord Drug Targets. 5 Dec 2016. [Epub ahead of print]
Liguori C, Placidi F, Albanese M et al (2014) CSF beta-amyloid levels are altered in narcolepsy: a link with the inflammatory hypothesis? J Sleep Res 23(4):420–424
Duits FH, Teunissen CE, Bouwman FH et al (2014) The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement 10:713–723
Liguori C, Romigi A, Mercuri NB et al (2014) Cerebrospinal-fluid orexin levels and daytime somnolence in frontotemporal dementia. J Neurol 261(9):1832–1836
Liguori C, Placidi F, Izzi F et al (2016) Beta-amyloid and phosphorylated tau metabolism changes in narcolepsy over time. Sleep Breath 20(1):277–283
Alessandrini M, Micarelli A, Chiaravalloti A et al (2014) Cerebellar metabolic involvement and its correlations with clinical parameters in vestibular neuritis. J Neurol 261(10):1976–1985
Della Rosa PA, Cerami C, Gallivanone F et al (2014) A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 12(4):575–593
Bennett CM, Wolford GL, Miller MB (2009) The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci 4(4):417–422
Lancaster JL, Rainey LH, Summerlin JL et al (1997) Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp 5(4):238–242
Liguori C, Chiaravalloti A, Sancesario G et al (2016) Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 43(11):2040–2049
Soonawala D, Amin T, Ebmeier KP et al (2002) Statistical parametric mapping of (99 m)Tc-HMPAO-SPECT images for the diagnosis of Alzheimer’s disease: normalizing to cerebellar tracer uptake. Neuroimage 17(3):1193–1202
Pagani M, De Carli F, Morbelli S et al (2014) Volume of interest-based [18F]fluorodeoxyglucose PET discriminates MCI converting to Alzheimer’s disease from healthy controls A European Alzheimer’s Disease Consortium (EADC) study. Neuroimage Clin 7:34–42
Lee DS, Kang H, Kim H et al (2008) Metabolic connectivity by interregional correlation analysis using statistical parametric mapping (SPM) and FDG brain PET; methodological development and patterns of metabolic connectivity in adults. Eur J Nucl Med Mol Imaging 35(9):1681–1691
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19(3):1233–1239
Cross DJ, Anzai Y, Petrie EC et al (2013) Loss of olfactory tract integrity affects cortical metabolism in the brain and olfactory regions in aging and mild cognitive impairment. J Nucl Med 54(8):1278–1284
Friedman LF, Zeitzer JM, Lin L et al (2007) Alzheimer disease, increased wake fragmentation found in those with lower hypocretin-1. Neurology 68(10):793–794
Naismith SL, Lewis SJ, Rogers NL (2011) Sleep-wake changes and cognition in neurodegenerative disease. Prog Brain Res 190:21–52
Prinz PN, Vitaliano PP, Vitiello MV et al (1982) Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging 3(4):361–370
Herholz K (2003) PET studies in dementia. Ann Nucl Med 17(2):79–89
Mosconi L (2013) Glucose metabolism in normal aging and Alzheimer’s disease: methodological and physiological considerations for PET studies. Clin Transl Imaging. doi:10.1007/s40336-013-0026-y
Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C (2003) Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage. 20(3):1894–1898
Shan L, Dauvilliers Y, Siegel JM (2015) Interactions of the histamine and hypocretin systems in CNS disorders. Nat Rev Neurol 11(7):401–413
Lu J, Sherman D, Devor M, Saper CB (2006) A putative flip-flop switch for control of REM sleep. Nature 441(7093):589–594
Konadhode RR, Pelluru D, Blanco-Centurion C et al (2013) Optogenetic stimulation of MCH neurons increases sleep. J Neurosci 33(25):10257–10263
Dauvilliers Y, Comte F, Bayard S et al (2010) A brain PET study in patients with narcolepsy–cataplexy. J Neurol Neurosurg Psychiatry 81(3):344–348
Huang YS, Liu FY, Lin CY et al (2016) Brain imaging and cognition in young narcoleptic patients. Sleep Med 24:137–144
Bozzali M, Padovani A, Caltagirone C, Borroni B (2011) Regional grey matter loss and brain disconnection across Alzheimer disease evolution. Curr Med Chem 18(16):2452–2458
Schmahmann JD, Doyon J, McDonald D et al (1999) Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10(3 Pt 1):233–260
Acknowledgements
We thank Prof. Carlo Chiaramonte for helping us in the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Claudio Liguori, Agostino Chiaravalloti, Nicola Biagio Mercuri, Marzia Nuccetelli, Giuseppe Sancesario, Francesca Izzi, Andrea Cimini, Sergio Bernardini, Orazio Schillaci, and Fabio Placidi declare no conflict of interest or financial disclosures.
Rights and permissions
About this article
Cite this article
Liguori, C., Chiaravalloti, A., Nuccetelli, M. et al. Hypothalamic dysfunction is related to sleep impairment and CSF biomarkers in Alzheimer’s disease. J Neurol 264, 2215–2223 (2017). https://doi.org/10.1007/s00415-017-8613-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8613-x