Abstract
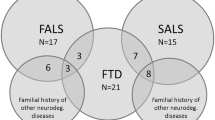
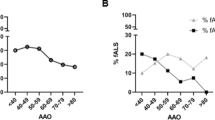
The C9orf72 repeat expansion (RE) is one of the most frequent causative mutations of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). However, it is still unclear how the C9orf72 RE can lead to a heterogeneous phenotype. Several reports have shown the coexistence of mutations in multiple ALS/FTD causative genes in the same family, suggesting an oligogenic etiology for ALS and FTD. Our aim was to investigate this phenomenon in an Italian group of ALS/FTD pedigrees carrying the C9orf72 RE. We included 11 subjects from 11 pedigrees with ALS/FTD and the C9orf72 RE. Mutation screening of FUS, SOD1 and TARDBP genes was performed by direct sequencing. A dementia-specific custom-designed targeted next-generation sequencing panel was used for screening dementia-associated genes mutations. We found genetic variants in additional ALS or dementia-related genes in four pedigrees, including the p.V47A variant in the TYROBP gene. As a group, double mutation carriers displayed a tendency toward a younger age at onset and a higher frequency of positive familiar history and of parkinsonism. Our observation supports the hypothesis that the co-presence of mutations in different genes may be relevant for the clinical expression of ALS/FTD and of their oligogenic nature.

Similar content being viewed by others
References
Bennion Callister J, Pickering-Brown SM (2014) Pathogenesis/genetics of frontotemporal dementia and how it relates to ALS. Exp Neurol 262:84–90
Renton AE, Chiò A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17:17–23
Majounie E, Renton AE, Mok K et al (2012) Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 11:323–330
Ferrari R, Mok K, Moreno JH, Cosentino S, Goldman J, Pietrini P, Mayeux R, Tierney MC, Kapogiannis D, Jicha GA, Murrell JR, Ghetti B, Wassermann EM, Grafman J, Hardy J, Huey ED, Momeni P (2012) Screening for C9ORF72 repeat expansion in FTLD. Neurobiol Aging 33:1850.e1–1850.e11
Galimberti D, Arosio B, Fenoglio C, Serpente M, Cioffi SM, Bonsi R, Rossi P, Abbate C, Mari D, Scarpini E (2014) Incomplete penetrance of the C9ORF72 hexanucleotide repeat expansions: frequency in a cohort of geriatric non-demented subjects. J Alzheimers Dis 39:19–22
Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C (2013) Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci 36:450–459
Beck J, Poulter M, Hensman D et al (2013) Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am J Hum Genet 92:345–353
Benussi L, Rossi G, Glionna M, Tonoli E, Piccoli E, Fostinelli S, Paterlini A, Flocco R, Albani D, Pantieri R, Cereda C, Forloni G, Tagliavini F, Binetti G, Ghidoni R (2015) C9ORF72 hexanucleotide repeat number in frontotemporal lobar degeneration: a genotype–phenotype correlation study. J Alzheimers Dis 45:319
Belzil VV, Katzman RB, Petrucelli L (2016) ALS and FTD: an epigenetic perspective. Acta Neuropathol 132:487–502
Cooper-Knock J, Kirby J, Highley R, Shaw PJ (2015) The spectrum of C9orf72-mediated neurodegeneration and amyotrophic lateral sclerosis. Neurotherapeutics 12:326–339
DeJesus-Hernandez M, Mackenzie IR et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256
Renton AE, Majounie E, Waite A et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS–FTD. Neuron 72:257–268
Lattante S, Ciura S, Rouleau GA, Kabashi E (2015) Defining the genetic connection linking amyotrophic lateral sclerosis (ALS) with frontotemporal dementia (FTD). Trends Genet 31:263–273
van Blitterswijk M, van Es MA, Hennekam EA, Dooijes D, van Rheenen W, Medic J, Bourque PR, Schelhaas HJ, van der Kooi AJ, de Visser M, de Bakker PI, Veldink JH, van den Berg LH (2012) Evidence for an oligogenic basis of amyotrophic lateral sclerosis. Hum Mol Genet 21:3776–3784
van Blitterswijk M, Baker MC, DeJesus-Hernandez M et al (2013) C9ORF72 repeat expansions in cases with previously identified pathogenic mutations. Neurology 81:1332–1341
Pastor P (2013) Comment: double mutants of frontotemporal dementia genes—simple co-occurrence? Neurology 81:1338
Ji AL, Zhang X, Chen WW, Huang WJ (2017) Genetics insight into the amyotrophic lateral sclerosis/frontotemporal dementia spectrum. J Med Genet 54:145–154
Rascovsky K, Hodges JR, Knopman D et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Discord 1:293–299
Hensman Moss DJ, Poulter M, Beck J, Hehir J, Polke JM, Campbell T, Adamson G, Mudanohwo E, McColgan P, Haworth A, Wild EJ, Sweeney MG, Houlden H, Mead S, Tabrizi SJ (2014) C9orf72 expansions are the most common genetic cause of Huntington disease phenocopies. Neurology 82:292–299
Visani A, de Biase D, Bartolomei I, Plasmati R, Morandi L, Cenacchi G, Salvi F, Pession A (2011) A novel T137A SOD1 mutation in an Italian family with two subjects affected by amyotrophic lateral sclerosis. Amyotroph Lateral Scler 12:385–388
Beck J, Pittman A, Adamson G, Campbell T, Kenny J, Houlden H, Rohrer JD, de Silva R, Shoai M, Uphill J, Poulter M, Hardy J, Mummery CJ, Warren JD, Schott JM, Fox NC, Rossor MN, Collinge J, Mead S (2014) Validation of next-generation sequencing technologies in genetic diagnosis of dementia. Neurobiol Aging 35:261–265
Bartoletti-Stella A, Chiaro G, Calandra-Buonaura G, Contin M, Scaglione C, Barletta G, Cecere A, Garagnani P, Tieri P, Ferrarini A, Piras S, Franceschi C, Delledonne M, Cortelli P, Capellari S (2015) A patient with PMP22-related hereditary neuropathy and DBH-gene-related dysautonomia. J Neurol 262:2373–2381
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL (2015) ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424
Schwarz JM, Rödelsperger C, Schuelke M, Seelow D (2010) MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7:575–576
Gamez J, Corbera-Bellalta M, Nogales G, Raguer N, García-Arumí E, Badia-Canto M, Lladó-Carbó E, Alvarez-Sabín J (2006) Mutational analysis of the Cu/Zn superoxide dismutase gene in a Catalan ALS population: should all sporadic ALS cases also be screened for SOD1? J Neurol Sci 247:21–28
Di Vito L, de Biase D, Pession A, Visani M, Liguori R, Zambito Marsala S, Leta V, De Carolis P, Donadio V (2013) Brachial amyotrophic diplegia associated with the a140a superoxide dismutase 1 mutation. Neurogenetics 14:255–256
Pottier C, Ravenscroft TA, Brown PH et al (2016) TYROBP genetic variants in early-onset Alzheimer’s disease. Neurobiol Aging 48:222.e9–222.e15
van der Zee J, Le Ber I, Maurer-Stroh S et al (2007) Mutations other than null mutations producing a pathogenic loss of progranulin in frontotemporal dementia. Hum Mutat 28:416
Guerreiro RJ, Washecka N, Hardy J, Singleton A (2010) A thorough assessment of benign genetic variability in GRN and MAPT. Hum Mutat 31:1126–1140
Das G, Sadhukhan T, Sadhukhan D, Biswas A, Pal S, Ghosh A, Das SK, Ray K, Ray J (2013) Genetic study on frontotemporal lobar degeneration in India. Parkinsonism Relat Disord 19:487–489
Puoti G, Lerza MC, Ferretti MG, Bugiani O, Tagliavini F, Rossi G (2014) A mutation in the 5′-UTR of GRN gene associated with frontotemporal lobar degeneration: phenotypic variability and possible pathogenetic mechanisms. J Alzheimers Dis 42:939–947
Chiò A, Restagno G, Brunetti M, Ossola I, Calvo A, Canosa A, Moglia C, Floris G, Tacconi P, Marrosu F, Marrosu MG, Murru MR, Majounie E, Renton AE, Abramzon Y, Pugliatti M, Sotgiu MA, Traynor BJ, Borghero G, SARDINIALS Consortium (2012) ALS/FTD phenotype in two Sardinian families carrying both C9ORF72 and TARDBP mutations. J Neurol Neurosurg Psychiatry 83:730–733
van Blitterswijk M, van Es MA, Koppers M, van Rheenen W, Medic J, Schelhaas HJ, van der Kooi AJ, de Visser M, Veldink JH, van den Berg LH (2012) VAPB and C9orf72 mutations in 1 familial amyotrophic lateral sclerosis patient. Neurobiol Aging 33:2950.e1–2950.e4
Lashley T, Rohrer JD, Mahoney C, Gordon E, Beck J, Mead S, Warren J, Rossor M, Revesz T (2014) A pathogenic progranulin mutation and C9orf72 repeat expansion in a family with frontotemporal dementia. Neuropathol Appl Neurobiol 40:502–513 (Erratum in: Neuropathol Appl Neurobiol 40:955)
Dekker AM, Seelen M, van Doormaal PT, van Rheenen W, Bothof RJ, van Riessen T, Brands WJ, van der Kooi AJ, de Visser M, Voermans NC, Pasterkamp RJ, Veldink JH, van den Berg LH, van Es MA (2016) Large-scale screening in sporadic amyotrophic lateral sclerosis identifies genetic modifiers in C9orf72 repeat carriers. Neurobiol Aging 39:220.e9–220.e15
Snowden JS, Adams J, Harris J, Thompson JC, Rollinson S, Richardson A, Jones M, Neary D, Mann DM, Pickering-Brown S (2015) Distinct clinical and pathological phenotypes in frontotemporal dementia associated with MAPT, PGRN and C9orf72 mutations. Amyotroph Lateral Scler Frontotemporal Degener 16:497–505
Rohrer JD, Ridgway GR, Modat M, Ourselin S, Mead S, Fox NC, Rossor MN, Warren JD (2010) Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage 53:1070–1076
Blumen SC, Inzelberg R, Nisipeanu P, Carasso RL, Oved D, Aizenstein O, Drory VE, Bergstrom C, Andersen PM (2010) Aggressive familial ALS with unusual brain MRI and a SOD1 gene mutation. Amyotroph Lateral Scler 11:228–231
Weber M, Neuwirth C, Thierbach J, Schweikert K, Czaplinski A, Petersen J, Jung HH, Birve A, Marklund SL, Andersen PM (2012) ALS patients with SOD1 mutations in Switzerland show very diverse phenotypes and extremely long survival. J Neurol Neurosurg Psychiatry 83:351–353
Keogh MJ, Wei W, Wilson I et al (2017) Genetic compendium of 1511 human brains available through the UK Medical Research Council Brain Banks Network Resource. Genome Res 27:165–173
Kiialainen A, Hovanes K, Paloneva J, Kopra O, Peltonen L (2005) Dap12 and Trem2, molecules involved in innate immunity and neurodegeneration, are co-expressed in the CNS. Neurobiol Dis 18:314–322
Painter MM, Atagi Y, Liu CC, Rademakers R, Xu H, Fryer JD, Bu G (2015) TREM2 in CNS homeostasis and neurodegenerative disease. Mol Neurodegener 10:43
Stanley ER, Chitu V (2014) CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 6:a021857
Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjaerg L, Konttinen Y, Peltonen L (2002) Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 71:656–662
Guerreiro RJ, Lohmann E, Brás JM, Gibbs JR, Rohrer JD, Gurunlian N, Dursun B, Bilgic B, Hanagasi H, Gurvit H, Emre M, Singleton A, Hardy J (2013) Using exon sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol 70:78–84
Guerreiro R, Wojtas A, Bras J et al (2013) TREM2 variants in Alzheimer’s disease. N Engl J Med 368:117–127
Giraldo M, Lopera F, Siniard AL, Corneveaux JJ, Schrauwen I, Carvajal J, Muñoz C, Ramirez-Restrepo M, Gaiteri C, Myers AJ, Caselli RJ, Kosik KS, Reiman EM, Huentelman MJ (2013) Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer’s disease. Neurobiol Aging 34:2077.e11–2077.e18
Rayaprolu S, Mullen B, Baker M et al (2013) TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener 8:19
Lynch DS, Jaunmuktane Z, Sheerin UM, Phadke R, Brandner S, Milonas I, Dean A, Bajaj N, McNicholas N, Costello D, Cronin S, McGuigan C, Rossor M, Fox N, Murphy E, Chataway J, Houlden H (2016) Hereditary leukoencephalopathy with axonal spheroids: a spectrum of phenotypes from CNS vasculitis to parkinsonism in an adult onset leukodystrophy series. J Neurol Neurosurg Psychiatry 87:512–519
Jonsson T, Stefansson H, Steinberg S et al (2013) Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368:107–116
Zhang B, Gaiteri C, Bodea LG et al (2013) Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153:707–720
Cady J, Koval ED, Benitez BA, Zaidman C, Jockel-Balsarotti J, Allred P, Baloh RH, Ravits J, Simpson E, Appel SH, Pestronk A, Goate AM, Miller TM, Cruchaga C, Harms MB (2014) TREM2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol 71:449–453
Radford RA, Morsch M, Rayner SL, Cole NJ, Pountney DL, Chung RS (2015) The established and emerging roles of astrocytes and microglia in amyotrophic lateral sclerosis and frontotemporal dementia. Front Cell Neurosci 9:414
Hooten KG, Beers DR, Zhao W, Appel SH (2015) Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics 12:364–375
O’Rourke JG, Bogdanik L, Yáñez A, Lall D, Wolf AJ, Muhammad AK, Ho R, Carmona S, Vit JP, Zarrow J, Kim KJ, Bell S, Harms MB, Miller TM, Dangler CA, Underhill DM, Goodridge HS, Lutz CM, Baloh RH (2016) C9orf72 is required for proper macrophage and microglial function in mice. Science 351:1324–1329
Ismail A, Cooper-Knock J, Highley JR, Milano A, Kirby J, Goodall E, Lowe J, Scott I, Constantinescu CS, Walters SJ, Price S, McDermott CJ, Sawcer S, Compton DA, Sharrack B, Shaw PJ (2013) Concurrence of multiple sclerosis and amyotrophic lateral sclerosis in patients with hexanucleotide repeat expansions of C9ORF72. J Neurol Neurosurg Psychiatry 84:79–87
Kantanen M, Kiuru-Enari S, Salonen O, Kaipainen M, Hokkanen L (2014) Subtle neuropsychiatric and neurocognitive changes in hereditary gelsolin amyloidosis (AGel amyloidosis). PeerJ 2:e493. doi:10.7717/peerj.493.eCollection
Heckman MG, Soto-Ortolaza AI, Sanchez Contreras MY et al (2016) LRRK2 variation and dementia with Lewy bodies. Parkinsonism Relat Discord 31:98–103
Kiely AP, Ling H, Asi YT, Kara E, Proukakis C, Schapira AH, Morris HR, Roberts HC, Lubbe S, Limousin P, Lewis PA, Lees AJ, Quinn N, Hardy J, Love S, Revesz T, Houlden H, Holton JL (2015) Distinct clinical and neuropathological features of G51D SNCA mutation cases compared with SNCA duplication and H50Q mutation. Mol Neurodegener 10:41
Pottier C, Bieniek KF, Finch N et al (2015) Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol 130:77–92
Petersen RB, Goren H, Cohen M, Richardson SL, Tresser N, Lynn A, Gali M, Estes M, Gambetti P (1997) Transthyretin amyloidosis: a new mutation associated with dementia. Ann Neurol 41:307–313
Acknowledgements
We are grateful to the patients and their families and to Miss Cecilia Baroncini for English editing and to Professor Kevin Talbot for his opinions on the manuscript. The authors gratefully acknowledge the Italian Ministry of Research RFO, Fondazione del Monte, Fondazione Gino Galletti for funding support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
Italian Ministry of Research RFO, Fondazione del Monte, Fondazione Gino Galletti to SC, PP, RL and AIRAlzh Onlus-COOP Italia to ABS.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standard
The study has been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Giannoccaro, M.P., Bartoletti-Stella, A., Piras, S. et al. Multiple variants in families with amyotrophic lateral sclerosis and frontotemporal dementia related to C9orf72 repeat expansion: further observations on their oligogenic nature. J Neurol 264, 1426–1433 (2017). https://doi.org/10.1007/s00415-017-8540-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8540-x