Abstract
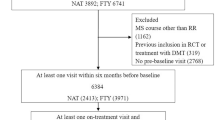
In this independent, multicentre post-marketing study we directly compared the effectiveness of natalizumab (NTZ), fingolimod (FNG) and self-injectable drugs (INJ), in non-responders to first immunomodulating treatment and in highly active treatment-naïve patients with multiple sclerosis. As main outcome measure we considered the proportions of patients with no evidence of disease activity (NEDA-3), defined as absence of relapses, disability worsening and radiological activity. A total of 567 non-responders to interferon beta (IFNB) or glatiramer acetate (GA) [dataset A] and 216 highly active treatment-naïves [dataset B] were followed up to 24 months from the beginning of NTZ, FNG or INJ, i.e. switching from IFNB to GA or viceversa (in the case of non-responders) or starting high-dose IFNB (in the case of highly active treatment-naïves). Propensity score matching in a 1:1:1 ratio was used to select only patients with similar baseline characteristics, retaining 330 and 120 patients in dataset A and B, respectively. In dataset A, the 24-month proportion with NEDA-3 was greater in both NTZ group (67%) and FNG group (42%) than in INJ group (35%) (p ≤ 0.016); however, NTZ was superior to FNG in promoting the attainment of NEDA-3 status (p = 0.034). In dataset B, the 24-month proportion with NEDA-3 was greater in NTZ group (75%) and FNG group (67%) than in INJ group (40%), but the small cohort sizes most likely prevented the detection of any statistically significant difference. Our study provides real-world evidence that NTZ was more effective than both FNG and INJ in non-responders, while it could seem that, in highly active treatment-naïves, NTZ was as effective as FNG and both were superior to INJ.




Similar content being viewed by others
References
Ransohoff RM, Hafler DA, Lucchinetti CF (2015) Multiple sclerosis-a quiet revolution. Nat Rev Neurol 11:134–142
Caon C, Din M, Ching W et al (2006) Clinical course after change of immunomodulating therapy in relapsing-remitting multiple sclerosis. Eur J Neurol 13:471–474
Carra´ A, Onaha P, Luetic G et al (2008) Therapeutic outcome 3 years after switching of immunomodulatory therapies in patients with relapsing-remitting multiple sclerosis in Argentina. Eur J Neurol 15:386–393
Limmroth V, Rolf M, Zettl UK et al (2007) Quality assessment in multiple sclerosis quality (QUASIMS): a comparison of different therapies for relapsing-remitting multiple sclerosis. J Neurol 254:67–77
Prosperini L, Borriello G, De Giglio L et al (2011) Management of breakthrough disease in patients with multiple sclerosis: when an increasing of Interferon beta dose should be effective? BMC Neurol 11:26
Prosperini L, Giannì C, Leonardi L et al (2012) Escalation to natalizumab or switching among immunomodulators in relapsing multiple sclerosis. Mult Scler 18:64–71
He A, Spelman T, Jokubaitis V et al (2015) Comparison of switch to fingolimod or interferon beta/glatiramer acetate in active multiple sclerosis. JAMA Neurol 72:405–413
Spelman T, Kalincik T, Zhang A et al (2015) Comparative efficacy of switching to natalizumab in active multiple sclerosis. Ann Clin Transl Neurol 2:373–387
Braune S, Lang M, Bergmann A, NTC Study Group (2016) Efficacy of fingolimod is superior to injectable disease modifying therapies in second-line therapy of relapsing remitting multiple sclerosis. J Neurol 263:327–333
Braune S, Lang M, Bergmann A, NTC Study Group (2013) Second line use of Fingolimod is as effective as Natalizumab in a German out-patient RRMS-cohort. J Neurol 260:2981–2985
Kalincik T, Horakova D, Spelman T et al (2015) Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol 77:425–435
Barbin L, Rousseau C, Jousset N et al (2016) Comparative efficacy of fingolimod vs natalizumab: a French multicenter observational study. Neurology 86:771–778
Baroncini D, Ghezzi A, Annovazzi PO et al (2016) Natalizumab versus fingolimod in patients with relapsing-remitting multiple sclerosis non-responding to first-line injectable therapies. Mult Scler 22:1315–1326
Koch-Henriksen N, Magyari M, Sellebjerg F, Soelberg Sørensen P (2016) A comparison of multiple sclerosis clinical disease activity between patients treated with natalizumab and fingolimod. Mult Scler (in press)
Signori A, Schiavetti I, Gallo F, Sormani MP (2015) Subgroups of multiple sclerosis patients with larger treatment benefits: a meta-analysis of randomized trials. Eur J Neurol 22:960–966
Ghezzi A, Grimaldi LM, Marrosu MG et al (2011) Natalizumab therapy of multiple sclerosis: recommendations of the Multiple Sclerosis Study Group-Italian Neurological Society. Neurol Sci 32:351–358
Filippi M, Rocca MA, Bastianello S et al (2013) Guidelines from The Italian Neurological and Neuroradiological Societies for the use of magnetic resonance imaging in daily life clinical practice of multiple sclerosis patients. Neurol Sci 34:2085–2093
Giovannoni G, Turner B, Gnanapavan S et al (2015) Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 4:329–333
Rotstein DL, Healy BC, Malik MT et al (2015) Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 72:152–158
Prosperini L, Fanelli F, Pozzilli C (2016) Long-term assessment of no evidence of disease activity with natalizumab in relapsing multiple sclerosis. J Neurol Sci 364:145–147
Rio J, Nos C, Tintoré M et al (2006) Defining the response to Interferon beta in relapsing-remitting Multiple Sclerosis patients. Ann Neurol 59:344–352
Altay EE, Fisher E, Jones SE et al (2013) Reliability of classifying multiple sclerosis disease activity using magnetic resonance imaging in a multiple sclerosis clinic. JAMA Neurol 70:338–344
Phillips JT, Giovannoni G, Lublin FD et al (2011) Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler 17:970–979
Trojano M, Pellegrini F, Paolicelli D (2009) Observational studies: propensity score analysis of non-randomized data. Int MS J 16:90–97
Rassen JA, Solomon DH, Glynn RJ, Schneeweiss S (2011) Simultaneously assessing intended and unintended treatment effects of multiple treatment options: a pragmatic “matrix design”. Pharmacoepidemiol Drug Saf 20:675–683
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107
Cummings P, McKnight B, Greenland S (2003) Matched cohort methods for injury research. Epidemiol Rev 25:43–50
Greenland S (1996) Basic methods for sensitivity analysis of biases. Int J Epidemiol 25:1107–1116
Kalincik T, Sormani MP (2016) Reporting treatment outcomes in observational data: a fine balance. Mult Scler. doi:10.1177/1352458516633902
Prosperini L, De Angelis F, De Angelis R et al (2015) Sustained disability improvement is associated with T1 lesion volume shrinkage in natalizumab-treated patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 86:236–238
Uitdehaag BM, Barkhof F, Coyle PK et al (2011) The changing face of multiple sclerosis clinical trial populations. Curr Med Res Opin 27:1529–1537
Montalban X (2011) Review of methodological issues of clinical trials in multiple sclerosis. J Neurol Sci 311(Suppl 1):S35–S42
Kappos L, Radue EW, O’Connor P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflict of interest.
Funding source
No external source of funding was required.
Financial disclosures
LP received consulting fees from Biogen and Novartis; speaker honoraria from Biogen, Genzyme, Novartis and Teva; travel Grants from Biogen, Genzyme, Novartis and Teva; research Grants from the Italian MS Society (Associazione Italiana Sclerosi Multipla) and Genzyme. He also acts as member of steering committee AIFA (Agenzia Italiana del Farmaco) on natalizumab. FS received personal compensation from Novartis, Forward Pharma, Almirall, Genzyme, and Teva for public speaking, editorial work and advisory boards. CC received consulting fees from Novartis and Merk Serono. AC ha nothing to disclose. FB received funding for traveling from Novartis, Teva, Merck Serono, Almirall, Biogen. SP received fees by Almirall, Biogen, Teva, and Genzyme and travel Grants by CSL Behring, Genzyme, Novartis, Teva, Kedrion. AB has nothing to disclose. SR received speaking honoraria from Merck Serono and Teva. SH has nothing to disclose. VBM received compensation for public speaking and advisory boards from Biogen, Merk Serono, Bayer, Genzyme, Almirall, Novartis, and Teva. RC received consulting fees from Novartis, Biogen and lecture fees and/or travel Grants from Novartis, Biogen, Genzyme and Sanofi-Aventis. DC acted as an Advisory Board member of Merck Serono, Teva, Bayer Schering, Biogen, Novartis, Almirall, GW Pharmaceuticals, Genzyme, Roche, and received funding for traveling and honoraria for speaking or consultation fees from Merck Serono, Teva, Novartis, Bayer Schering, Sanofi-Aventis, Biogen, Almirall, Genzyme. He also acts as member of steering committee AIFA (Agenzia Italiana del Farmaco) on natalizumab. GDB received speaker honoraria from Merck Serono, Biogen Idec, Sanofi-Aventis and Bayer Shering Pharma; received support for participation to National and International Congresses from Bayer-Schering, Biogen-Idec, Merck Serono, Novartis, Sanofi-Aventis and Teva. EF has nothing to disclose. AF received honoraria for lecturing and compensation for travel expenses from Merck Serono, Teva, Novartis, Bayer Schering, Sanofi-Aventis, and Biogen. SG has received fees as invited speaker or travel expenses for attending meeting from Biogen, Merck Serono, Teva, Almirall, Sanofi-Aventis, Novartis, Genzyme. CG received lecture fees and/or consulting fees from Merck Serono, Biogen, Teva, Bayer Schering and Novartis. EM received funding for traveling and speaking honoraria from Novartis and Teva. MM received honoraria from Biogen, Genzyme, Novartis, Merck Serono, Almirall and Teva. CP has received consulting and/or lecture fees and/or research funding and travel Grant from Almirall, Bayer Schering, Biogen, Genzyme, Merck Serono, Novartis, Roche and Teva.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prosperini, L., Saccà, F., Cordioli, C. et al. Real-world effectiveness of natalizumab and fingolimod compared with self-injectable drugs in non-responders and in treatment-naïve patients with multiple sclerosis. J Neurol 264, 284–294 (2017). https://doi.org/10.1007/s00415-016-8343-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8343-5