Abstract
Although fingolimod is registered in Europe for treatment of relapsing-remitting multiple sclerosis (RRMS) if earlier disease modifying therapy (DMT) has failed, no data regarding its efficacy in this patient group are available. This observational cohort study of the NeuroTransData network includes German RRMS outpatients with failure of earlier therapy with injectable DMT (iDMT), therefore switching to either another iDMT (n = 133) or to fingolimod (n = 300). Statistical comparison of clinical baseline characteristics showed more severely affected patients in the fingolimod group. A propensity-score matched group comparison was performed (n = 99 in each group) covering more than 2-year observation time. Fingolimod showed statistically significant superior efficacy in comparison to iDMT regarding annualized relapse rate (0.21 versus 0.33 per year), time-to-relapse and likelihood of relapse (iDMT hazard ratio 1.7), proportion and likelihood of patients with EDSS progression (15.10 versus 31.00 %; iDMT hazard ratio 1.7), persistence on medication and likelihood of discontinuation (iDMT hazard ratio 3.0). Significantly more patients were free of relapse and EDSS progression with fingolimod than with their second iDMT (64.4 versus 46.5 %, p < 0.03). This real-life evidence in German RRMS outpatients support data from controlled clinical studies and can quantitatively support clinical decision finding processes if iDMT therapy fails in RRMS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical studies of fingolimod leading to registration in Europe showed superiority in clinical and MRI parameters of RRMS patients in comparison to interferon-β-1a intramuscular (TRANSFORMS [1] and placebo (FREEDOMS [2]). Based on safety concerns the market authorization by EMA decided in March 2011 (ema.europa.eu/Find medicine/Human medicines/European Public Assessment Reports) that fingolimod should be given only if RRMS patients had failed to respond to at least one other disease modifying therapy or because their disease is getting worse rapidly. Until 2011 only natalizumab was available if injectable disease modifying therapies (iDMTs, Betaferon® interferon β-1b sc, Rebif® interferon β-1a sc, Avonex® interferon β-1a im, Copaxone® glatirameracetat, Extavia® interferon β-1b sc) failed with its specific benefit–risk profile associated with cases of progressive multifocal leukencephalopathy (PML) occurring since 2004. Therefore up to 79 % of RRMS patients switched within iDMTs in the US [3]. Although fingolimod offers a new treatment option since 2011 for patients failing on iDMT therapy, there is no known evidence regarding the efficacy of fingolimod in this particular clinical situation. This observational cohort study investigates the course of RRMS patients with the failure of iDMT treatment, who either switched within iDMT or to fingolimod.
Methods
This is an observational cohort study using health data routinely collected in outpatient neurology practices throughout Germany who are members of the NeuroTransData (NTD) network. Beside demographic data clinical parameters like relapses, EDSS and medication are documented digitally in-time during clinical visits at least once within 3-month periods in all patients with MS. All neurologists are certified EDSS-rater. All participating medical staff are trained to document these data in-time in a standardized way in the web-based digital NTD data source. This data acquisition protocol is approved by the ethical committee of the Bavarian Medical Board (Bayerische Landesärztekammer, 14.06.2012). The data are pooled anonymously to form the database of the study.
This cohort analysis includes:
-
RRMS patients with failure of iDMT therapy as judged by the treating neurologist and the patient between 01.01.2010 and 30.06.2015,
-
who switched either to another iDMT medication or to fingolimod and,
-
who had a documented observation period of a minimum of 180 days.
The decision to switch and the choice of treatment were at the discretion of the treating neurologist and the patient.
The primary outcome parameters were EDSS progression, relapse rate and adherence to medication. Progression of EDSS was defined as an increase of the EDSS score by one point if baseline EDSS was smaller than 5.5, or 0.5 points if baseline EDSS was equal or higher than 5.5.
Time-to-event analysis using Kaplan–Meyer survival curves were calculated for time-to-progression, time-to relapse and adherence including hazard ratios. Proportions of patients free of progression and/or relapses were calculated.
Patient population
The NTD database identified 1,472 RRMS patients switching therapy. 433 of them fulfilled all inclusion criteria with 300 patients switching to fingolimod and 133 within iDMT. 59.5 % of the 1,472 patients, almost completely switching within iDMT, had to be excluded because the reason to switch was not treatment failure but others, like adverse events. Comparing baseline characteristics of clinical parameters of the fingolimod- and iDMT-cohort statistical analysis identified significant differences as shown in Table 1.
In this German outpatient cohort, RRMS patients switching to fingolimod after failure of earlier iDMT therapy had a significantly longer MS duration, showed higher EDSS baseline scores, higher relapse rate and higher proportion of patient suffering EDSS progression in the previous year than patients switching within iDMT.
To enable a comparison of efficacy a propensity-score matching was performed to define comparable cohort groups. Patients were propensity score matched on EDSS score and 3 months confirmed EDSS score when starting on second therapy, number of relapses in the 360 days before starting on second therapy and years since diagnosis of RRMS. Variables considered for the propensity score model but not included were: gender, region of birth, age, and presence of relapse in the 361–720 days period before starting second therapy (Table 2).
Results
Persistence on medication
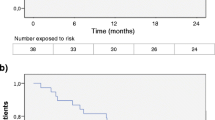
Persistence on medication was significantly better in the cohort with fingolimod compared to iDMT already from the very beginning of therapy with persistence after 1 year for fingolimod 95 % and iDMT 70 %, after 2 years 85 and 56 %, respectively (Fig. 1). Insufficient efficacy in about 11 times as many and side effects in about 50 % more patients with iDMT therapy than with fingolimod were causing discontinuation (Table 3).
Relapse rate
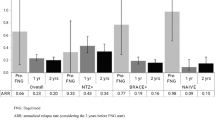
Annualized relapse rate and time-to-relapse analyses were statistically significantly in favour of fingolimod (Figs. 2, 3, 4).
EDSS progression
The number of patients showing EDSS progression during the observation time was significantly lower (p = 0.0231) in the fingolimod cohort (n = 11; 15.10 %) than in the iDMT cohort (n = 22; 31.00 %) (Tables 4, 5).
Cox proportional hazard models for matched patients
Hazard ratios were higher for the cohort on iDMT compared to fingolimod, reaching statistical significance for persistence on medication and relapse.
Freedom of clinical disease activity: NEDA 2
The proportion of patients with/without evidence of clinical MS disease activity regarding EDSS progression and/or relapse was analyzed.
During treatment with fingolimod significantly more patients remained free of relapses and EDSS progression and fewer patients suffered from relapses and EDSS progression.
Discussion
This outpatient observational cohort study of German RRMS patients demonstrates superiority of therapy with fingolimod after failure of an earlier iDMT therapy regarding persistence on medication, relapse rate and EDSS progression compared to another medication within iDMT therapies. Significantly more patients were free of relapses and EDSS progression when treated with fingolimod compared to iDMT. This results in an impressively better persistence on fingolimod medication than on iDMT. The hazard ratios for patients switching within iDMT showed a threefold risk for discontinuation of medication and 1.7-fold risks for relapses and EDSS progression compared to fingolimod. In our matched populations the hazard ratio for discontinuation of iDMT compared to fingolimod was even higher than in previously published unmatched groups (NTD cohort hazard ratio 3.044 for iDMT versus glatirameracetat 1.75, interferon-1β 2.01 in [4]).
Overall data indicate that efficacy of fingolimod from controlled studies can be replicated in real-life regarding freedom of EDSS progression after 12 months [NTD: fingolimod 88 %, iDMT 80 %; TRANSFORM [1]: fingolimod 94 %, interferon β-1a (IFβ-1a) 92 %], and annualized relapse rate (NTD: fingolimod 0.21, iDMT 0.33; TRANSFORM [1]: fingolimod 0.16, IFβ-1a 0.33). Differences of results between this NTD cohort study and the TRANSFORM study reflect that the NTD cohort included RRMS patients with an unfavourable course during earlier iDMT therapy, while TRANSFORMS with IFβ-1a as control group included patients independent of previous course and medication.
These results can quantitatively support decision finding processes in individual RRMS patients if iDMT therapy fails. Accumulating evidence showing good cardiac safety of fingolimod even in patients with preexisting cardiac conditions [5] supports the benefit–risk considerations. Enduring persistence on medication based on clinical efficacy associated with good tolerability and safety leads to a cost-effective allocation of health system resources in favour of fingolimod compared to other DMTs [6, 7].
The impact of recent reports on PML in two patients with fingolimod with no prior exposure to immunosuppressant drugs on the benefit–risk ratio of fingolimod is under discussion as specific risk factors remain to be identified and PML seems to be associated with a number of MS immunoactive drugs.
References
Cohen JA, Barkhof Frederik, Comi G, Hartung H-P, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Stacy W, Aradhye S, Kappos L, The TRANSFORMS Study Group (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Kappos L, Radue E-W, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Bonafede MM, Johnson BH, Wenten M, Watson C (2013) Treatment patterns in disease-modifying therapy for patients with multiple sclerosis in the United States. Clin Ther 35(10):1501–1512
Bergvall N, Petrilla AA, Karkare SU, Lahoz R, Agashivala N, Pradhan A, Capkun G, Makin C, McGuiness CB, Korn JR (2014) Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ 17(10):696–707
Gold R, Comi G, Palace J, Siever A, Gottschalk R, Bijarnia M, von Rosenstiel P, Tomic D, Kappos L, FIRST Study Investigators (2014) Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: a phase 3b, open-label study. J Neurol 261(2):267–276
Agashivala N, Kim E (2012) Cost-effectiveness of early initiation of fingolimod versus delayed initiation after 1 year of intramuscular interferon beta-1a in patients with multiple sclerosis. Clin Ther 34(7):1583–1590
Brandes DW, Raimundo K, Agashivala N, Kim E (2013) Implications of real-world adherence on cost-effectiveness analysis in multiple sclerosis. J Med Econ 16(4):547–551
Acknowledgments
This cohort study was not financially supported by any institution or sponsor. All members of the NTD study group have received honoraria from almost all pharmaceutical companies in the fields of neurology and psychiatry for lecturing, consultancy or participation in clinical studies.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard
The data acquisition protocol of this cohort study is approved by the ethical committee of the Bavarian Medical Board (Bayerische Landesärztekammer, 14.06.2012) and have been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Braune, S., Lang, M., Bergmann, A. et al. Efficacy of fingolimod is superior to injectable disease modifying therapies in second-line therapy of relapsing remitting multiple sclerosis. J Neurol 263, 327–333 (2016). https://doi.org/10.1007/s00415-015-7970-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7970-6