Abstract
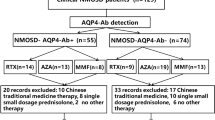
Rituximab, a chimeric monoclonal anti-CD20 antibody, has been proposed to be effective for neuromyelitis optica spectrum disorder (NMOSD). A concern for developing progressive multifocal leukoencephalopathy (PML), which is caused by John Cunningham virus (JCV), has been suggested particularly in patients treated long term with rituximab. In this study, using a modified enzyme-linked immunosorbent assay with glutathione S-transferase-tagged VP1 as the antigen, we investigated the seroprevalence of anti-JCV antibodies among 78 Korean patients with NMOSD and the change in anti-JCV antibody serostatus following long-term rituximab treatment. The overall seroprevalence of anti-JCV antibodies was 69 % prior to rituximab administration. Over a mean of 4 years of repeated treatment with rituximab, no patient developed PML. Of 24 initially seronegative patients, none converted into seropositive, whereas six (11 %) of 54 initially seropositive patients converted into seronegative. Our results might support the safety of long-term rituximab treatment in patients with NMOSD with regard to the risk of developing PML.

Similar content being viewed by others
References
Jacob A, Weinshenker BG, Violich I, McLinskey N, Krupp L, Fox RJ, Wingerchuk DM, Boggid M, Constantinescu CS, Miller A, De Angelis T, Matiello M, Cree BA (2008) Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch Neurol 65(11):1443–1448
Kim SH, Huh SY, Lee SJ, Joung A, Kim HJ (2013) A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol 70(9):1110–1117
Stroopinsky D, Katz T, Rowe JM, Melamed D, Avivi I (2012) Rituximab-induced direct inhibition of T-cell activation. Cancer Immunol Immunother 61(8):1233–1241
Liossis SN, Sfikakis PP (2008) Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol 127(3):280–285
van de Veerdonk FL, Lauwerys B, Marijnissen RJ, Timmermans K, Di Padova F, Koenders MI, Gutierrez-Roelens I, Durez P, Netea MG, van der Meer JW, van den Berg WB, Joosten LA (2011) The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum 63(6):1507–1516
Carson KR, Evens SM, Richey EA, Habermann TM, Focosi D, Seymour JF, Laubach J, Bawn SD, Gordon LI, Winter JN, Furman RR, Vose JM, Zelenetz AD, Mamtani R, Raisch DW, Dorshimer GW, Rosen ST, Muro K, Gottardi-Littell NR, Talley RL, Sartor O, Green D, Major EO, Bennett CL (2009) Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports projects. Blood 113(20):4834–4840
Clifford DB, Ances B, Costello C, Rosen-Schmidt S, Andersson M, Parks D, Perry A, Yerra R, Schmidt R, Alvarez E, Tyler KL (2011) Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol 68(9):1156–1164
Gorelik L, Lerner M, Bixler S, Crossman M, Schlain B, Simon K, Pace A, Cheung A, Chen LL, Berman M, Zein F, Wilson E, Yednock T, Sandrock A, Goelz SE, Subramanyam M (2010) Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 68(3):295–303
Warnke C, Pawlita M, Dehmel T, Posevitz-Fejfar A, Hartung HP, Wiendl H, Kieseier BC, Adams O (2013) An assay to quantify species-specific anti-JC virus antibody levels in MS patients. Mult Scler 19(9):1137–1144
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366(20):1870–1880
Carruthers RL, Rotstein DL, Healy BC, Chitnis T, Weiner HL, Buckle GJ (2014) An observational comparison of natalizumab vs. fingolimod using JCV serology to determine therapy. Mult Scler 20(10):1381–1390
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66(10):1485–1489
Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG (2007) The spectrum of neuromyelitis optica. Lancet Neurol 6(9):805–815
Antonsson A, Green AC, Mallitt KA, O’Rourke PK, Pawlita M, Waterboer T, Neale RE (2010) Prevalence and stability of antibodies to the BK and JC polyomaviruses: a long-term longitudinal study of Australians. J Gen Virol 91(Pt 7):1849–1853
Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH (2009) Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 199(6):837–846
Warnke C, Ramanujam R, Plavina T, Bergström T, Goelz S, Subramanyam M, Kockum I, Rahbar A, Kieseier BC, Holmén C, Olsson T, Hillert J, Fogdell-Hahn A (2013) Changes to anti-JCV antibody levels in a Swedish national MS cohort. J Neurol Neurosurg Psychiatry 84(11):1199–1205
Outteryck O, Zephir H, Salleron J, Ongagna JC, Etxeberria A, Collongues N, Lacour A, Fleury MC, Blanc F, Giroux M, de Seze J, Vermersch P (2013) JC-virus seroconversion in multiple sclerosis patients receiving natalizumab. Mult Scler (Epub ahead of print)
Trampe AK, Hemmelmann C, Stroet A, Haghikia A, Hellwig K, Wiendl H, Goelz S, Ziegler A, Gold R, Chan A (2012) Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort. Neurology 78(22):1736–1742
Kim W, Kim SH, Huh SY, Kong SY, Choi YJ, Cheong Hj, Kim HJ (2013) Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. Eur J Neurol 20(6):975–980
Oren S, Mandelboim M, Braun-Moscovici Y, Paran D, Ablin J, Litinsky I, Comaneshter D, Levartovsky D, Mendelson E, Azar R, Wigler I, Balbir-Gurman A, Caspi D, Elkayam O (2008) Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis 67(7):937–941
Acknowledgments
The author(s) received no specific funding for this work.
Conflicts of interest
Kim SH, Hyun JW, Jeong IH, Joung AR, Yeon JL and Adams O report no conflict of interest. Dehmel T has received compensation for travel expenses from Novartis Pharma and Genzyme. Kieseier BC has received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Bayer Health Care, Biogen Idec, Genzyme/Sanofi Aventis, Grifols, Merck Serono, Mitsubishi Europe, Novartis, Roche, Talecris, and Teva. Kim HJ has received honoraria for speaking or consulting from Bayer Schering Pharma, Biogen Idec, Genzyme, Merck Serono, Novartis, MedImmune, and Teva-Handok and has received research grants from Genzyme, Merck Serono, and Kael-GemVax.
Ethical standard
This study was approved by the Institutional Review Board of the National Cancer Center, and informed consent was obtained from all patients.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SH., Hyun, JW., Jeong, I.H. et al. Anti-JC virus antibodies in rituximab-treated patients with neuromyelitis optica spectrum disorder. J Neurol 262, 696–700 (2015). https://doi.org/10.1007/s00415-014-7629-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7629-8