Abstract
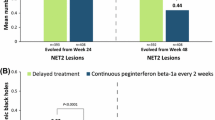
Conversion of active lesions to black holes has been associated with disability progression in subjects with relapsing–remitting multiple sclerosis (RRMS) and represents a complementary approach to evaluating clinical efficacy. The objective of this study was to assess the conversion of new active magnetic resonance imaging (MRI) lesions, identified 6 months after initiating treatment with glatiramer acetate 40 mg/mL three-times weekly (GA40) or placebo, to T1-hypointense black holes in subjects with RRMS. Subjects received GA40 (n = 943) or placebo (n = 461) for 12 months. MRI was obtained at baseline and Months 6 and 12. New lesions were defined as either gadolinium-enhancing T1 or new T2 lesions at Month 6 that were not present at baseline. The adjusted mean numbers of new active lesions at Month 6 converting to black holes at Month 12 were analyzed using a negative binomial model; adjusted proportions of new active lesions at Month 6 converting to black holes at Month 12 were analyzed using a logistic regression model. Of 1,292 subjects with complete MRI data, 433 (50.3 %) GA-treated and 247 (57.2 %) placebo-treated subjects developed new lesions at Month 6. Compared with placebo, GA40 significantly reduced the mean number (0.31 versus 0.45; P = .0258) and proportion (15.8 versus 19.6 %; P = .006) of new lesions converting to black holes. GA significantly reduced conversion of new active lesions to black holes, highlighting the ability of GA40 to prevent tissue damage in RRMS.




Similar content being viewed by others
References
Rovira A, Auger C, Alonso J (2013) Magnetic resonance monitoring of lesion evolution in multiple sclerosis. Ther Adv Neurol Disord 6:298–310
Zivadinov R, Bakshi R (2004) Role of MRI in multiple sclerosis II: brain and spinal cord atrophy. Front Biosci 9:647–664
Filippi M, Rocca MA, Camesasca F et al (2011) Interferon β-1b and glatiramer acetate effects on permanent black hole evolution. Neurology 76:1222–1228
Filippi M, Rovaris M, Rocca MA, Sormani MP, Wolinsky JS, Comi G (2001) Glatiramer acetate reduces the proportion of new MS lesions evolving into “black holes”. Neurology 57:731–733
Filippi M, Preziosa P, Rocca MA (2014) Magnetic resonance outcome measures in multiple sclerosis trials: time to rethink? Curr Opin Neurol 27:290–299
Cadavid D, Cheriyan J, Skurnick J, Lincoln JA, Wolansky LJ, Cook SD (2009) New acute and chronic black holes in patients with multiple sclerosis randomised to interferon beta-1b or glatiramer acetate. J Neurol Neurosurg Psychiatry 80:1337–1343
Sahraian MA, Radue EW, Haller S, Kappos L (2010) Black holes in multiple sclerosis: definition, evolution, and clinical correlations. Acta Neurol Scand 122:1–8
Zivadinov R, Stosic M, Cox JL, Ramasamy DP, Dwyer MG (2008) The place of conventional MRI and newly emerging MRI techniques in monitoring different aspects of treatment outcome. J Neurol 255(Suppl 1):61–74
Barkhof F, Karas GB, van Walderveen MA (2000) T1 hypointensities and axonal loss. Neuroimaging Clin N Am 10:739–752 (ix)
Cadavid D, Wolansky LJ, Skurnick J et al (2009) Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 72:1976–1983
Nagtegaal GJ, Pohl C, Wattjes MP et al (2014) Interferon beta-1b reduces black holes in a randomised trial of clinically isolated syndrome. Mult Scler 20:234–242
Zivadinov R, Hussein S, Bergsland N, Minagar A, Dwyer MG (2012) Magnetization transfer imaging of acute black holes in patients on glatiramer acetate. Front Biosci (Elite Ed) 4:1496–1504
Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R, Group GS (2013) Three times weekly glatiramer acetate in relapsing–remitting multiple sclerosis. Ann Neurol 73:705–713
Zivadinov R, Rudick RA, De Masi R et al (2001) Effects of IV methylprednisolone on brain atrophy in relapsing–remitting MS. Neurology 57:1239–1247
Polman CH, Reingold SC, Edan G et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58:840–846
Filippi M, Rocca MA, Pagani E et al (2014) Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J Neurol Neurosurg Psychiatry 85:851–858
Arnold DL, Gold R, Kappos L et al (2014) Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study. J Neurol 261:1794–1802
Fox RJ, Miller DH, Phillips JT et al (2012) Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 367:1087–1097
Bagnato F, Jeffries N, Richert ND et al (2003) Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain 126:1782–1789
Acknowledgments
We thank the patients and site personnel involved with this study and Peter Feldman, PhD (Teva Pharmaceuticals), and Lisa Grauer, MSc (Chameleon Communications International with funding and writing/editorial support from Teva Pharmaceutical Industries), for editorial assistance in the preparation of this report. This study was funded by Teva Pharmaceutical Industries Ltd, Petach Tikva, Israel.
Conflicts of interest
R. Zivadinov: personal compensation from Teva, Biogen Idec, EMD Serono, Genzyme-Sanofi, Claret Medical, Novartis for speaking and consultant fees; financial support for research activities from Biogen Idec, Teva, Novartis, Genzyme-Sanofi, Claret Medical, Greatbatch.
M. Dwyer: consulting fees from Claret Medical, EMD Serono.
J. Steinerman, V. Knappertz, H. Barkay: employees of Teva Pharmaceutical Industries.
O. Khan: research funding from NIH, NINDS, NMSS, Teva, Biogen Idec, Genzyme, Roche, Novartis; consulting fees from Biogen Idec, Genzyme, Novartis; speaker bureaus for Teva, Novartis, Biogen Idec.
Ethical standard
All institutional review boards or ethics committees of the particpating centers approved the protocol and all patients gave written, informed consent before any study-related procedures were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zivadinov, R., Dwyer, M., Barkay, H. et al. Effect of glatiramer acetate three-times weekly on the evolution of new, active multiple sclerosis lesions into T1-hypointense “black holes”: a post hoc magnetic resonance imaging analysis. J Neurol 262, 648–653 (2015). https://doi.org/10.1007/s00415-014-7616-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7616-0