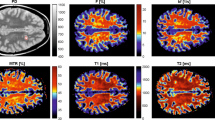
Abstract
Magnetic resonance imaging (MRI) is the most important paraclinical measure for assessing and monitoring the pathologic changes implicated in the onset and progression of multiple sclerosis (MS). Conventional MRI sequences, such as T1-weighted gadolinium (Gd) enhanced and spin-echo T2-weighted imaging, only provide an incomplete picture of the degree of inflammation and underlying neurodegenerative changes in this disease. Two- and three-dimensional fluid-attenuated inversion recovery and double inversion recovery sequences allow better identification of cortical, periventricular and infratentorial lesions. Ultra-high field strength MRI has the potential to detect subpial cortical and deep gray matter lesions. Unenhanced T1-weighted imaging can reveal hypointense black holes, a measure of chronic neurodegeneration. Magnetization transfer imaging (MTI) is increasingly used to characterize the evolution of MS lesions and normal-appearing brain tissue. Evidence suggests that the dynamics of magnetization transfer changes correlate with the extent of demyelination and remyelination. Magnetic resonance spectroscopy, which provides details on tissue biochemistry, metabolism, and function, also has the capacity to reveal neuroprotective mechanisms. By measuring the motion of water, diffusion imaging can provide information about the orientation, size, and geometry of tissue damage in white and gray matter. These advanced non-conventional MRI techniques relate better to clinical impairment, disease progression, accumulation of disability, and have the potential to detect neuroprotective effects of treatment. Although detecting the status of neuronal integrity using MRI techniques continues to improve, a “gold standard” model remains to be established.
Similar content being viewed by others
References
Anderson VM, Fox NC, Miller DH (2006) Magnetic resonance imaging measures of brain atrophy in multiple sclerosis. J Magn Reson Imaging 23:605–618
Arnold DL, Matthews PM, Francis GS, O'Connor J, Antel JP (1992) Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol 31:235–241
Barkhof F, Bruck W, De Groot CJ, Bergers E, Hulshof S, Geurts J, Polman CH, van der Valk P (2003) Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch Neurol 60:1073–1081
Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, Comi G, Ader HJ, Losseff N, Valk J (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120(Pt 11):2059–2069
Barkhof F, Karas GB, van Walderveen MA (2000) T1 hypointensities and axonal loss. Neuroimaging Clinics of North America 10:739–752, ix
Bedell BJ, Narayana PA (1998) Implementation and evaluation of a new pulse sequence for rapid acquisition of double inversion recovery images for simultaneous suppression of white matter and CSF. J Magn Reson Imaging 8:544–547
Bermel RA, Bakshi R (2006) The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 5:158–170
Bink A, Schmitt M, Gaa J, Mugler JP 3rd, Lanfermann H, Zanella FE (2006) Detection of lesions in multiple sclerosis by 2D FLAIR and single-slab 3D FLAIR sequences at 3.0 T: initial results. Eur Radiol 16:1104–1110
Bo L, Geurts JJ, van der Valk P, Polman C, Barkhof F (2007) Lack of correlation between cortical demyelination and white matter pathologic changes in multiple sclerosis. Arch Neurol 64:76–80
Cadavid C, Lincoln J, Baguero A, Segal L, Gomez-Choco M, Alamani J, Peng B, Tullock K, Joseph G, Szczepanowski K, Skurnick J, Halper J, Vidgop Y, Baladandapani P, Cook S, Wolansky L (2006) Outcome of T1 hypointensities in patients with early forms of multiple sclerosis randomized to Betaseron or Copaxone and followed by monthly 3T MRI for up to 2 years: preliminary analysis of the BECOME study. Mult Scler 12(Suppl 1):S97
Chen JT, Collins DL, Atkins HL, Freedman MS, Galal A, Arnold DL (2006) Brain atrophy after immunoablation and stem cell transplantation in multiple sclerosis. Neurology 66:1935–1937
Chen JT, Collins DL, Freedman MS, Atkins HL, Arnold DL (2005) Local magnetization transfer ratio signal inhomogeneity is related to subsequent change in MTR in lesions and normal-appearing white matter of multiple sclerosis patients. NeuroImage 25:1272–1278
Chen JT, Kuhlmann T, Jansen GH, Collins DL, Atkins HL, Freedman MS, O’Connor PW, Arnold DL (2007) Voxel-based analysis of the evolution of magnetization transfer ratio to quantify remyelination and demyelination with histopathological validation in a multiple sclerosis lesion. NeuroImage 36:1152–1158
Comi G, Filippi M, Wolinsky JS (2001) European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging – measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol 49:290–297
Cotton F, Weiner HL, Jolesz FA, Guttmann CR (2003) MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology 60:640–646
Dalton CM, Chard DT, Davies GR, Miszkiel KA, Altmann DR, Fernando K, Plant GT, Thompson AJ, Miller DH (2004) Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain 127:1101–1107
Deloire-Grassin MS, Brochet B, Quesson B, Delalande C, Dousset V, Canioni P, Petry KG (2000) In vivo evaluation of remyelination in rat brain by magnetization transfer imaging. J Neurol Sci 178:10–16
Dwyer M, Dolezal O, Hussein S, Horakova D, Havrdova E, Cox J, Zivadinov R (2007) Development of central atrophy may lead to underestimation of lesion accrual in patients with multiple sclerosis. ISMRM
Erskine MK, Cook LL, Riddle KE, Mitchell JR, Karlik SJ (2005) Resolution-dependent estimates of multiple sclerosis lesion loads. The Can J Neurol Sci 32:205–212
Filippi M (2000) Enhanced magnetic resonance imaging in multiple sclerosis. Mult Scler 6:320–326
Filippi M, Rovaris M, Capra R, Gasperini C, Prandini F, Martinelli V, Horsfield MA, Bastianello S, Sormani MP, Pozzilli C, Comi G (1999) Interferon beta treatment for multiple sclerosis has a graduated effect on MRI enhancing lesions according to their size and pathology. J Neurol Neurosurg Psychiatry 67:386–389
Filippi M, Rovaris M, Capra R, Gasperini C, Yousry TA, Sormani MP, Prandini F, Horsfield MA, Martinelli V, Bastianello S, Kuhne I, Pozzilli C, Comi G (1998) A multi-centre longitudinal study comparing the sensitivity of monthly MRI after standard and triple dose gadolinium-DTPA for monitoring disease activity in multiple sclerosis. Implications for phase II clinical trials. Brain 121(Pt 10):2011–2020
Filippi M, Rovaris M, Inglese M, Barkhof F, De Stefano N, Smith S, Comi G (2004) Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet 364:1489–1496
Filippi M, Yousry T, Rocca MA, Fesl G, Voltz R, Comi G (1997) Sensitivity of delayed gadolinium-enhanced MRI in multiple sclerosis. Acta Neurol Scand 95:331–334
Ge Y, Grossman RI, Udupa JK, Fulton J, Constantinescu CS, Gonzales-Scarano F, Babb JS, Mannon LJ, Kolson DL, Cohen JA (2000) Glatiramer acetate (Copaxone) treatment in relapsing-remitting MS: quantitative MR assessment. Neurology 54:813–817
Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA (2005) Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 236:254–260
Hardmeier M, Wagenpfeil S, Freitag P, Fisher E, Rudick RA, Kooijmans M, Clanet M, Radue EW, Kappos L (2005) Rate of brain atrophy in relapsing MS decreases during treatment with IFNbeta-1a. Neurology 64:236–240
Horakova D, Cox JL, Havrdova E, Hussein S, Dolezal O, Cookfair D, Dwyer MG, Seidl Z, Bergsland N, Vaneckova M, Zivadinov R (2007) Evolution of different MRI measures in patients with active relapsing-remitting multiple sclerosis over 2 and 5 years. A case control study. J Neurol Neurosurg Psychiatry
Inglese M (2006) Multiple sclerosis: new insights and trends. AJNR 27:954–957
Inglese M, Mancardi GL, Pagani E, Rocca MA, Murialdo A, Saccardi R, Comi G, Filippi M (2004) Brain tissue loss occurs after suppression of enhancement in patients with multiple sclerosis treated with autologous haematopoietic stem cell transplantation. J Neurol Neurosurg Psychiatry 75:643–644
Jones C, Riddehough A, Li D, Zhao G, Paty D (2001) MRI cerebral atrophy in relapsing-remitting MS: results from the PRISMS trial. Neurology 56(Suppl 3):A379
Kangarlu A, Bourekas EC, Ray-Chaudhury A, Rammohan KW (2007) Cerebral cortical lesions in multiple sclerosis detected by MR imaging at 8 Tesla. AJNR 28:262–266
Kappos L, Moeri D, Radue EW, Schoetzau A, Schweikert K, Barkhof F, Miller D, Guttmann CR, Weiner HL, Gasperini C, Filippi M (1999) Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet 353:964–969
Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Bauer L, Jakobs P, Pohl C, Sandbrink R (2006) Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 67:1242–1249
Khan O, Mackenzie M, Shen Y, Zak I, Latif Z, Caon C (2007) Combined Brain MTR and H-MRS Multi-Modality Approach To Investigate Mechanism of Action of Interferon Beta and Glatiramer Acetate in RRMS. Neurology 68:A57
Khan O, Shen Y, Hu J, Ching W, Caon C, Reznar M, Latif Z, Tselis A, Lisak R (2005) Sustained effect of glatiramer acetate on cerebral axonal recovery in relapsing-remitting MS: results after three years of serial brain magnetic resonance spectroscopy examination. J Neurol 252:127–128
Kutzelnigg A, Faber-Rod JC, Bauer J, Lucchinetti CF, Sorensen PS, Laursen H, Stadelmann C, Bruck W, Rauschka H, Schmidbauer M, Lassmann H (2007) Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol (Zurich, Switzerland) 17:38–44
Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712
Lincoln J, Belenguer A, Vidgop E, Cadavid D, Wolansky L, Skurnick J, Cook S (2007) Comparison of Betaseron and Copaxone on newly enhancing lesions by monthly 3T MRI with triple dose gadolinium: secondary outcomes in a 15-month analysis of the BECOME study. Neurology 68(Suppl 1):A331
Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F (2007) Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130:1089–1104
Narayanan S, Caramanos Z, Arnold D (2004) The effect of glatiramer acetate treatment on axonal integrity in multiple sclerosis. Mult Scler 10:S256
Narayanan S, De Stefano N, Francis GS, Arnaoutelis R, Caramanos Z, Collins DL, Pelletier D, Arnason BGW, Antel JP, Arnold DL (2001) Axonal metabolic recovery in multiple sclerosis patients treated with interferon beta-1b. J Neurol 248:979–986
Parry A, Corkill R, Blamire AM, Palace J, Narayanan S, Arnold D, Styles P, Matthews PM (2003) Beta-Interferon treatment does not always slow the progression of axonal injury in multiple sclerosis. J Neurol 250:171–178
Pascual-Lozano A, Martinez-Bisbal M, Bosca I, Valero C, Coret F, Martinez-Granados B, Marti-Bonmati L, Celda B, Casanova B (2006) Axonal damage and inflammation in early multiple sclerosis: effects of subcutaneous interferon-beta-1a treatment. Mult Scler 12(Suppl 1):S186
Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Bruck W, Lucchinetti C, Lassmann H (2006) Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129:3165–3172
Rademacher J, Engelbrecht V, Burgel U, Freund H, Zilles K (1999) Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer MR. NeuroImage 9:393–406
Ramasamy D, Fritz D, Cox J, Abdelrahman N, Hussein S, Dwyer M, Zivadinov R (2007) Extent of deep grey matter atrophy in patients with multiple sclerosis. A case control study. Mult Scler 13(Suppl 2):P601; S180
Rocca MA, Filippi M (2007) Functional MRI in multiple sclerosis. J Neuroimaging 17(Suppl 1):36S–41S
Roccatagliata L, Rocca MA, Valsasina P, Bonzano L, Sormani MP, Saccardi R, Mancardi GL, Filippi M (2007) The long-term effect of AHSCT on MRI measures of MS evolution: a five-year follow-up study. Mult Scler 13:1068–1070
Rovaris M, Codella M, Moiola L, Ghezzi A, Zaffaroni M, Mancardi G, Capello E, Sardanelli F, Comi G, Filippi M (2002) Effect of glatiramer acetate on MS lesions enhancing at different gadolinium doses. Neurology 59:1429–1432
Rovira A, Alonso J, Cucurella G, Nos C, Tintore M, Pedraza S, Rio J, Montalban X (1999) Evolution of multiple sclerosis lesions on serial contrast-enhanced T1-weighted and magnetization-transfer MR images. AJNR 20:1939–1945
Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L (1999) Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology 53:1698–1704
Sarchielli P, Presciutti O, Tarducci R, Gobbi G, Alberti A, Pelliccioli GP, Orlacchio A, Gallai V (1998) 1H-MRS in patients with multiple sclerosis undergoing treatment with interferon beta-1a: results of a preliminary study. J Neurol Neurosurg Psychiatry 64:204–212
Sicotte NL, Voskuhl RR, Bouvier S, Klutch R, Cohen MS, Mazziotta JC (2003) Comparison of multiple sclerosis lesions at 1.5 and 3.0 Tesla. Invest Radiol 38:423–427
Silver NC, Good CD, Barker GJ, MacManus DG, Thompson AJ, Moseley IF, McDonald WI, Miller DH (1997) Sensitivity of contrast enhanced MRI in multiple sclerosis. Effects of gadolinium dose, magnetization transfer contrast and delayed imaging. Brain 120(Pt 7):1149–1161
Silver NC, Good CD, Sormani MP, MacManus DG, Thompson AJ, Filippi M, Miller DH (2001) A modified protocol to improve the detection of enhancing brain and spinal cord lesions in multiple sclerosis. J Neurol 248:215–224
Simmons ML, Frondoza CG, Coyle JT (1991) Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience 45:37–45
Sormani MP, Rovaris M, Valsasina P, Wolinsky JS, Comi G, Filippi M (2004) Measurement error of two different techniques for brain atrophy assessment in multiple sclerosis. Neurology 62:1432–1434
Stone LA, Frank JA, Albert PS, Bash C, Smith ME, Maloni H, McFarland HF (1995) The effect of interferon-beta on blood-brain barrier disruptions demonstrated by contrast-enhanced magnetic resonance imaging in relapsing-remitting multiple sclerosis. Ann Neurol 37:611–619
van Waesberghe JH, van Walderveen MA, Castelijns JA, Scheltens P, Lycklama a Nijeholt GJ, Polman CH, Barkhof F (1998) Patterns of lesion development in multiple sclerosis: longitudinal observations with T1-weighted spin-echo and magnetization transfer MR. AJNR 19:675–683
Wattjes MP, Harzheim M, Kuhl CK, Gieseke J, Schmidt S, Klotz L, Klockgether T, Schild HH, Lutterbey GG (2006) Does high-field MR imaging have an influence on the classification of patients with clinically isolated syndromes according to current diagnostic MR imaging criteria for multiple sclerosis? AJNR 27:1794–1798
Wattjes MP, Lutterbey GG, Gieseke J, Traber F, Klotz L, Schmidt S, Schild HH (2007) Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis lesions. AJNR 28:54–59
Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH (1999) Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 52:1626–1632
Wolansky L, Cook S, Skurnick J, Lincoln J, Tulloch K, Franco P, Haghighi M, Peng B, Lebovitz Y, Petscavage J, Szczepanowski K, Cadavid D (2007) Betaseron vs. Copaxone in MS with triple-dose gadolinium and 3-T MRI endpoints (BECOME): announcement of final primary study outcome. Mult Scler 13(Suppl 2):S58
Wolansky L, Cook S, Skurnick J, Tullock K, Joseph G, Sheynzon V, Bhaghat N, Haghighi M, Halper J, Cadavid C (2006) Betaseron vs Copaxone in multiple sclerosis with triple dose gadolinium and 3-T MRI endpoints (BECOME): efficacy of the optimized MRI protocol and announcement of primary study outcome. Mult Scler 12(Suppl 1):S98
Wolinsky J (2005) MRI as a surrogate. Mult Scler 11:S1–S82
Zivadinov R (2007) Can imaging techniques measure neuroprotection and remyelination in multiple sclerosis? Neurology 68:S72–S82; discussion S91–S76
Zivadinov R (2007) Role of MRI in imaging of myelin. Mult Scler 13(Suppl 2):S13
Zivadinov R, Bakshi R (2004) Central nervous system atrophy and clinical status in multiple sclerosis. J Neuroimaging 14:27S–35S
Zivadinov R, Bakshi R (2004) Role of MRI in multiple sclerosis I: inflammation and lesions. Front Biosci 9:665–683
Zivadinov R, Fritz D, Hani N, Nussenbaum F, Weinstock-Guttman B, Durfee J, Abdelrahman N, Hussein N, De Brujin M, Cox J, Dwyer M (2007) Voxel-wise dynamic classification of new, stable, resolving and atrophied T2 hyperintense lesion volumes in patients with multiple sclerosis. A 2-year longitudinal study. Mult Scler (in press)
Zivadinov R, Hussein S, Abdelrahman N, Cookfair D, Meyer M, Garg N, Cox J, Dwyer M, Weinstock-Guttman B (2006) Effect of glatiramer acetate on diffusion imaging in patients with multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England) 12(Suppl 1):S99
Zivadinov R, Leist TP (2005) Clinical-magnetic resonance imaging correlations in multiple sclerosis. J Neuroimaging 15:10S–21S
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zivadinov, R., Stosic, M., Cox, J.L. et al. The place of conventional MRI and newly emerging MRI techniques in monitoring different aspects of treatment outcome. J Neurol 255 (Suppl 1), 61–74 (2008). https://doi.org/10.1007/s00415-008-1009-1
Issue Date:
DOI: https://doi.org/10.1007/s00415-008-1009-1