Abstract
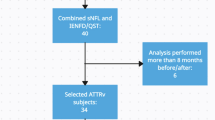
Individuals carrying the amyloidogenic transthyretin (TTR) mutation are predisposed to develop familial amyloid polyneuropathy (FAP). The first clinical manifestations are often subjective sensory symptoms and, therefore, objective markers are needed to confirm the onset of TTR-FAP. The purpose of this study was to evaluate the usefulness of a comprehensive battery of neurophysiological tests to provide early markers of FAP in TTR-mutation carriers. Twenty patients with documented pathogenic TTR mutation were enrolled, including eight clinically asymptomatic carriers and 12 paucisymptomatic carriers who had subjective sensory symptoms or doubtful sensory signs but no firm clinical deficit at clinical examination. Neurophysiological assessment of large fibres consisted of conventional nerve conduction studies. Small-fibre tests included laser evoked potentials, sympathetic skin responses and the measurement of cold and warm detection thresholds and heart-rate variability. Abnormalities in small-fibre tests were found in two of the eight asymptomatic carriers and nine of the 12 paucisymptomatic carriers. In contrast, conventional conduction studies did not show any sign of polyneuropathy. Early neuropathic involvement in TTR-mutation carriers can be detected or confirmed by neurophysiological tests specifically assessing small nerve fibres. These tests did not show any redundancy or difference in their sensitivity, emphasizing the value of combining them for TTR-FAP diagnosis.

Similar content being viewed by others
References
Planté-Bordeneuve V, Said G (2011) Familial amyloid polyneuropathy. Lancet Neurol 10(12):1086–1097
Holmgren G et al (1993) Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet 341(8853):1113–1116
Stangou AJ, Hawkins PN (2004) Liver transplantation in transthyretin-related familial amyloid polyneuropathy. Curr Opin Neurol 17(5):615–620
Coelho T et al (2012) Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 79(8):785–792
Said G, Grippon S, Kirkpatrick P (2012) Tafamidis. Nat Rev Drug Discov 11(3):185–186
Alves M, Conceição I, Luis ML (1997) Neurophysiological evaluation of sexual dysfunction in familial amyloidotic polyneuropathy–Portuguese type. Acta Neurol Scand 96(3):163–166
Shivji ZM, Ashby P (1999) Sympathetic skin responses in hereditary sensory and autonomic neuropathy and familial amyloid neuropathy are different. Muscle Nerve 22(9):1283–1286
Kim DH et al (2009) Quantitative sensation and autonomic test abnormalities in transthyretin amyloidosis polyneuropathy. Muscle Nerve 40(3):363–370
Heldestad V, Nordh E (2007) Quantified sensory abnormalities in early genetically verified transthyretin amyloid polyneuropathy. Muscle Nerve 35(2):189–195
Conceição IM et al (2008) Neurophysiological markers in familial amyloid polyneuropathy patients: early changes. Clin Neurophysiol 119(5):1082–1087
Fruhstorfer H, Lindblom U, Schmidt WC (1976) Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry 39(11):1071–1075
Said G (2003) Small fiber involvement in peripheral neuropathies. Curr Opin Neurol 16(5):601–602
Periquet MI et al (1999) Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology 53(8):1641–1647
Devigili G et al (2008) The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 131(Pt 7):1912–1925
Løseth S et al (2008) Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol 255(8):1197–1202
Scherens A et al (2009) Painful or painless lower limb dysesthesias are highly predictive of peripheral neuropathy: comparison of different diagnostic modalities. Eur J Pain 13(7):711–718
Shun CT et al (2004) Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 127(Pt 7):1593–1605
Lund C et al (2007) Histopathological and clinical findings in leprosy patients with chronic neuropathic pain: a study from Hyderabad, India. Lepr Rev 78(4):369–380
Vlckova-Moravcova E et al (2008) Small-fibre involvement in diabetic patients with neuropathic foot pain. Diabet Med 25(6):692–699
Løseth S et al (2010) Polyneuropathy in type 1 and type 2 diabetes: comparison of nerve conduction studies, thermal perception thresholds and intraepidermal nerve fibre densities. Diabetes Metab Res Rev 26(2):100–106
Nebuchennykh M, Løseth S, Mellgren SI (2010) Aspects of peripheral nerve involvement in patients with treated hypothyroidism. Eur J Neurol 17(1):67–72
Ragé M et al (2011) Asymptomatic small fiber neuropathy in diabetes mellitus: investigations with intraepidermal nerve fiber density, quantitative sensory testing and laser-evoked potentials. J Neurol 258(10):1852–1864
Nebuchennykh M et al (2009) The value of skin biopsy with recording of intraepidermal nerve fiber density and quantitative sensory testing in the assessment of small fiber involvement in patients with different causes of polyneuropathy. J Neurol 256(7):1067–1075
Casanova-Molla J et al (2011) On the relationship between nociceptive evoked potentials and intraepidermal nerve fiber density in painful sensory polyneuropathies. Pain 152(2):410–418
Chao CC et al (2010) Pathophysiology of neuropathic pain in type 2 diabetes: skin denervation and contact heat-evoked potentials. Diabetes Care 33(12):2654–2659
Ragé M et al (2010) The time course of CO2 laser-evoked responses and of skin nerve fibre markers after topical capsaicin in human volunteers. Clin Neurophysiol 121(8):1256–1266
Lefaucheur JP, Créange A (2004) Neurophysiological testing correlates with clinical examination according to fibre type involvement and severity in sensory neuropathy. J Neurol Neurosurg Psychiatry 75(3):417–422
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest
Dr. Lefaucheur has received speaker honoraria from Pfizer-Synergy and LFB and serves on the editorial board of the journals Clinical Neurophysiology and Neurophysiologie Clinique/Clinical Neurophysiology. Dr. Ng Wing Tin reports no disclosures. Dr. Kerschen reports no disclosures. Dr. Damy has received speaker honoraria from Pfizer-Synergy. Dr. Planté-Bordeneuve has received speaker honoraria from Pfizer-Synergy and as clinical investigator in Fx1A-301 study.
Ethical standard
All human studies must state that they have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lefaucheur, JP., Ng Wing Tin, S., Kerschen, P. et al. Neurophysiological markers of small fibre neuropathy in TTR-FAP mutation carriers. J Neurol 260, 1497–1503 (2013). https://doi.org/10.1007/s00415-012-6816-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6816-8