Abstract
Background
Recent evidence suggests that both serum neurofilament light chain (sNfL) levels and small fiber related diagnostic variables may be valuable disease biomarkers of hereditary transthyretin amyloidosis with polyneuropathy (ATTRv-PN).
Our study aimed to explore the relations between sNfL and small fiber related skin biopsy and quantitative sensory testing (QST) parameters in a cohort of ATTRv-PN patients and pre-symptomatic carriers.
Methods
We retrospectively analyzed data from 13 ATTRv patients and 21 pre-symptomatic carriers who underwent sNfL dosage, skin biopsy, and QST, and analyzed correlations between sNFL, intraepidermal nerve fiber density (IENFD), and cold (CDT) and warm detection thresholds (WDT).
Results
Both sNfL and small fiber related parameters significantly differed between carriers and patients (sNfL: p < 0.0001; IENFD: p = 0.0008; CDT, WDT: < 0.0001). sNFL levels were normal in all carriers, altered in 85% of patients, negatively correlated with distal IENFD (r = -0.47, p = 0.005), and significantly correlated with CDT (r = -0.68; p < 0.0001) and WDT (r = 0.57; p < 0.0001).
Conclusions
Our study showed that sNfL reliably discriminates symptomatic ATTRv-PN patients from pre-symptomatic carriers, and found significant relations between sNfL, skin biopsy, and QST small fiber related parameters, suggesting that sNfL might be a valuable biomarker of peripheral nerve involvement in ATTRv-PN and a supportive criterion for symptomatic disease transition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hereditary transthyretin amyloidosis (ATTRv, v for ‘variant’) is an adult-onset, autosomal-dominant disease, caused by pathogenic variants in the TTR gene, encoding the transthyretin protein. Diffuse extracellular deposition of misfolded amyloidogenic mutant TTR causes multi-organ damage, with prevalent hearth and peripheral somatic and autonomic nerves involvement, leading to disability and death if left untreated [1]. Recently, the management of ATTRv has radically changed, thanks to the development of several therapeutic molecules able to delay or even prevent disease progression [2,3,4,5]. Since these therapies are greatly effective if early started in the disease course, efficient biomarkers of disease onset are increasingly needed [6].
In the last few years, small nerve fiber damage, as assessed by skin biopsy [6,7,8,9,10,11,12,13,14] and quantitative sensory testing (QST) [15,16,17], has been shown at early stages in ATTRv with polyneuropathy (ATTRv-PN), even preceding the onset of symptoms in pre-symptomatic carriers [14, 17]. At the same time, recent studies suggested that serum neurofilament light chain (sNfL), axonal structural compounds released during nerve degeneration, may help discriminate symptomatic patients from pre-symptomatic TTR mutation carriers [18, 19], thus representing a sensitive, non-invasive, and easily repeatable biomarker of disease onset [20,21,22,23,24,25,26].
Our study aimed to investigate the relations between sNfL, lately identified as promising disease biomarker, and small fiber related variables, which are well-known early impaired indicators of neural damage in the disease course. To do so, we retrospectively analyzed data from a cohort of ATTRv pre-symptomatic carriers and symptomatic patients who underwent both sNfL, skin biopsy, and QST assessment.
Methods
Subjects’ selection
We conducted a retrospective analysis of data from ATTRv patients and pre-symptomatic carriers participating to our two multicentric studies about small fiber dysfunction characterization through skin biopsy and QST [14, 17], who underwent sNfL dosage at different disease stages [18]. Subjects were recruited at our amyloidosis referral Centers (Sant’ Andrea and Agostino Gemelli University Hospitals) between January 2020 and November 2022, and were considered eligible if they carried a known amyloidogenic variant in TTR and were older than 18 years. Subjects with other known causes or risk factors for peripheral neuropathy, such as diabetes, alcohol abuse, vitamin B12 deficiency, paraproteinemia, or hypothyroidism, were excluded [18]. We selected subjects who underwent both nerve conduction study, skin biopsy/QST evaluation, and sNfL dosage in a timespan of ± 8 months (Fig. 1). We furtherly divided the study population into pre-symptomatic carriers and symptomatic patients. We considered as carriers those subjects with no sensory or vegetative symptoms, normal neurological examination with a Neuropathy Impairment Scale (NIS) of 0–1 [27], and normal sural sensory nerve action potential (SNAP) amplitude accordingly with our laboratories’ normal values. Conversely, subjects who reported at least two symptoms at the Small Fiber Neuropathy Symptoms Inventory Questionnaire (SFN-SIQ) [28] and presented a NIS ≥ 2 were considered as symptomatic patients [13], regardless the amplitude of sural SNAP. To avoid excluding patients with pure small fiber neuropathy from the study cohort, we did not incorporate a reduction in sural sensory nerve action potential (SNAP) as a criterion for symptomatic patients’ selection. Thus, symptomatic patients could exhibit either normal or impaired sural SNAP. Patients with normal sural SNAP fulfilled Besta criteria for small fiber neuropathy, based on clinical examination, QST, and skin biopsy findings [29]. Symptoms and signs associated with neurophysiological evidence of carpal tunnel syndrome (CTS) or following the median nerve's distribution were excluded from the calculation of SFN-SIQ and NIS scores. We exclusively included symptoms and signs indicative of polyneuropathy, characterized by a length-dependent or symmetrical distribution, in the determination of SFN-SIQ symptoms and NIS signs. Symptomatic patients were sub-classified using the polyneuropathy disability (PND) scoring system [30]. All subjects were routinely evaluated in our Centers by expert cardiologists to disclose any possible hearth involvement. Cardiac involvement was diagnosed in all individuals with increased interventricular septum thickness (> 13 mm) by echocardiography and/or the presence of amyloid deposits in the heart as assessed by Tc-99 m HMDP bone scintigraphy [31].
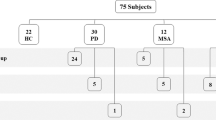
Subjects’ selection flow-chart. 140 ATTRv subjects were screened. From those 40 subjects who underwent both sNfL dosage, QST, and skin biopsy, we excluded 6 who had performed the procedures with a time-gap of more than 8 months before/after. We finally selected 34 ATTRv subjects, furtherly classified as pre-symptomatic carriers (n = 21) and symptomatic patients (n = 13)
Small nerve fiber assessment
Skin biopsy sampling, immunofluorescence staining protocol for protein gene product 9.5 (PGP 9.5) and collagen type IV, as well as intraepidermal nerve fiber density (IENFD) determination, were performed as previously reported [32]. Distal IENFD was considered as the main outcome skin biopsy variable using both raw values [33] and percentage reduction with respect to the lower cut-off of age-sex adjusted normative values [34].
QST was performed following the standardized protocol of the German Research Network on Neuropathic Pain [35]. The following QST small fiber related parameters were evaluated: cold and warm detection thresholds (CDT and WDT); cold and heat pain thresholds (CPT and HPT); mechanical pain threshold (MPT). A z-score was calculated for each QST variable (z-score = value of the patient—mean value of control subjects/standard deviation of control subjects) [36]. CDT and WDT were considered as main QST small fiber related outcome variables [37].
sNfL measurement
Blood samples were collected into serum separator tubes, clotted for 30 min at room temperature and then centrifuged at 1000 g for 15 min. Aliquots were stored at − 80 °C until analysis. sNfL concentration was measured using the Simple Plex™ cartridge-based assay on the Ella™ platform (ProteinSimple, San Jose, CA, USA), according to the manufacturer's instructions. A cut-off of 37.10 pg/mL was considered for normalcy, as previously reported [26].
Statistical analysis
A preliminary univariate analysis was performed to describe the main demographic and diagnostic test variables in pre-symptomatic carriers’ and patients’ group.
Since the normal distribution was rejected for all the considered continuous variables, as verified with the Kolmogorov–Smirnov test, quantitative variables were expressed as their median with interquartile range (IQR) and were compared between the two groups by the non-parametric Mann–Whitney U test. Correlations between the main outcome variables were verified through the Spearman’s correlation test. Simple linear regression models were used to assess linear relations between selected correlated variables.
Fisher’s exact test was used to compare the frequency of sNfL levels and small fiber related variables impairment between the two groups. For each variable, diagnostic specificity and sensitivity for the symptomatic disease state were calculated. Where applicable, simple logistic regression models were fitted to calculate the diagnostic accuracy of sNfL levels and small fiber related variables, as independent variables, to predict the presence of symptomatic disease state, as dependent variable.
No adjustment for multiple comparisons was adopted since all comparisons were pre-planned and our a priori intent was to test each variable independently. A p-value lower than 0.05 was considered significant. Analyses and graphics were performed with GraphPad Prism 10 software.
Ethics approval
This protocol was approved by Sapienza University Ethical Committee (UNTTR-19–1, protocol number 275 SA_2019 19/12/2019) and Ethical Committee of Fondazione Policlinico Agostino Gemelli IRCSS (Prot. ID 4108). It was carried out according to the principles of the 1964 Declaration of Helsinki. Each enrolled subject provided a written informed consent, and all study data were obtained and elaborated in accordance with our institutional ethical committee regulations.
Results
Clinical and demographic variables
Subjects’ selection flow-chart is reported in Fig. 1. Briefly, 140 ATTRv subjects were screened. From those 40 subjects who underwent both sNfL dosage and small nerve fiber assessment, we excluded 6 who had performed the procedures with a time-gap of more than 8 months before/after. Finally, we selected 34 ATTRv subjects, furtherly classified as pre-symptomatic carriers (n = 21) and symptomatic patients (n = 13). Demographic and clinical variables of the two study cohorts are reported in Table 1. As expected, age differed significantly between patients and pre-symptomatic carriers. The p.Val50Met mutation was more represented in pre-symptomatic subjects, probably due to larger familiar clusters studied in our geographical area [30]. Most evaluated patients were classified as PND1, the milder stage of disease-related disability. Most patients showed a mixed phenotype, with both cardiac and neuropathic involvement, whereas 3 were cardiac prevalent and 2 neuropathic prevalent. All symptomatic patients had both increased interventricular septum thickness and heart amyloid deposit at the bone scintigraphy except from those three with neuropathic prevalent phenotype. All PND2 and PND3 patients were under treatment with Patisiran at the time of the evaluation. Out of 8 PND1 subjects, 5 were under disease-modifying treatments (2 Tafamidis, 2 Inotersen, 1 Patisiran).
Diagnostic test alterations distribution
Both sNfL levels and small fiber related parameters (distal IENFD, CDT, WDT, HPT, CPT, and MPT) significantly differed between pre-symptomatic and symptomatic subjects, as shown in Table 1. Sural SNAP was reduced or absent in 9 out of 13 (69%) of symptomatic patients. Symptomatic patients with normal sural SNAP fulfilled Besta criteria for small fiber neuropathy [29].
Considering a cut-off value of 37.10 pg/mL [26], sNfL levels were normal in all pre-symptomatic subjects and altered in 11/13 (85%) of symptomatic patients. Of the two symptomatic patients with normal sNfL levels, one had a cardiac prevalent phenotype (sNfL 28.4 pg/mL), the other one a mild mixed phenotype (sNfL 26.7 pg/mL). Fisher’s exact test showed that sNfL levels abnormalities could distinguish carriers from patients with a sensitivity of 84% and a specificity of 100% (p < 0.0001). A simple logistic regression model, with sNfL levels as independent variable and the presence of symptomatic disease state as dependent variable, showed that sNfL could significantly predict the disease state with an AUC of 0.99 (p < 0.0001).
IENFD was reduced in 9/22 (41%) of pre-symptomatic carriers and in 10/13 (77%) of symptomatic subjects. The main small fiber related QST outcome variables (WDT and/or CDT) were impaired in 4/21 (19%) of asymptomatic carriers and in 7/13 (54%) of symptomatic patients. Fisher’s exact test showed that the presence of IENFD reduction and of the main small fiber related QST variables abnormalities could not discriminate pre-symptomatic carriers from symptomatic patients.
Correlations between sNfL and diagnostic variables
sNfL negatively correlated with distal IENFD (r = -0.47, p = 0.0050) and significantly correlated with the main small fiber related QST parameters impairment (CDT: r = -0.68, p < 0.0001; WDT: r = 0.57, p = 0.0005; HPT: r = 0.6, p = 0.0100; CPT: r = 0.44, p = 0.0002).
Simple linear regression models showed a linear relation between sNfL and distal IENFD (r2 = 0.3, p = 0.0009), CDT (r2 = 0.4, p = 0.0001), WDT (r2 = 0.5, p < 0.0001), and sural SNAP (r2 = 0.4, p < 0.0001) (Fig. 2). Given the strict linear relation between sNfL and age (r2 = 0.5, p < 0.0001), we also developed linear regression models using the percentage of distal IENFD reduction respect to the lower cut-off of age-sex adjusted normative values (r2 = 0.2, p < 0.003), as well as the z-scores of CDT and WDT (r2 = 0.2, p < 0.003 and r2 = 0.2, p < 0.01 respectively), showing conserved linear relations between these variables (Fig. 2).
Linear relations between sNfL levels and the main small fiber related variables. Simple linear regression models showing a linear relation between sNfL and distal IENFD (A), WDT (C), CDT (E), and sural SNAP (H). Given the strict linear relation between sNfL and age (G), we also developed linear regression models using the percentage of axonal loss (B), i.e., the distal IENFD reduction respect to the lower cut-off of age-sex adjusted normative values, as well as the z-scores of WDT (D) and CDT (F), showing conserved linear relations between these variables. Legend: sNfL: serum neurofilament light chain levels; IENFD: intraepidermal nerve fiber density at the distal site; WDT: warm detection threshold; CDT: cold detection threshold; sural SNAP: sural sensory nerve action potential
Discussion
In our study we showed that sNfL reliably discriminates symptomatic ATTRv-PN patients from pre-symptomatic carriers, and found significant relations between sNfL, skin biopsy, and QST small fiber related parameters, suggesting that sNfL might be a valuable biomarker of peripheral nerve involvement in ATTRv-PN and a supportive criterion for symptomatic disease transition.
The therapeutic landscape of ATTRv deeply changed in the last decade, due to the advent of new effective disease modifying therapies (DMTs). Moreover, it has been demonstrated that the earlier a DMT is started, the better the functional outcomes are [38]. In this scenario, the development and validation of reliable biomarkers of disease onset are crucial to implement tempestive DMT initiation, avoiding inappropriate prescriptions.
Previous studies have shown significant changes in sNfL levels [20,21,22,23,24] and small nerve fiber function and morphometry [7,8,9,10,11,12,13,14,15,16,17], as assessed by skin biopsy and QST, since the earliest disease stages in several ATTRv-PN populations. However, no study has investigated the relation between sNfL and small fiber related parameters in ATTRv-PN to our knowledge.
NfL are structural compounds contributing to maintain mitochondrial and microtubule stability of neuronal soma and axons at both central and peripheral nervous system level, with increasing evidence demonstrating their rise in different nerve degeneration conditions, including polyneuropathies of various etiologies [39,40,41,42].
Small fiber damage has traditionally been considered a hallmark of ATTRv-PN and has been early detected even in pre-symptomatic mutations carriers with skin biopsy and QST thermal thresholds assessment, the most widely agreed tools to assess small fiber morphometry and function [37, 43, 44]. In our cohort, small fiber related parameters abnormalities were unable to reliably distinguish pre-symptomatic from symptomatic subjects, thus showing a low specificity for the disease state condition; in fact, consistently with previous data [17, 45], distal IENFD and QST thermal thresholds resulted to be frequently early impaired also in pre-symptomatic carriers, with 41% showing distal IENFD reduction and 19% QST abnormalities of CDT and/or WDT.
Conversely, in our study sNfL levels abnormalities could predict the presence of symptomatic disease with very high sensitivity (85%) and specificity (100%), with all pre-symptomatic carriers showing normal sNfL levels (< 37.10 pg/mL). Since sNfL increase in peripheral neuropathies has been linked to peripheral nerve damage acuteness and progression [41, 42], we could speculate that small fiber damage, even if early detectable at skin biopsy and QST, occurs at such a mild rate and degree in pre-symptomatic subjects that it doesn’t induce an appreciable rise of sNfL, until a hypothetical threshold is reached when both neuropathic symptoms and sNfL changes begin. In this respect, sNfL rise could represent a supportive criterion to diagnose symptomatic transition in ATTRv carriers, in addition to the traditional small fiber related diagnostic parameters like distal IENFD and QST thermal thresholds, whose specificity is lowered by early abnormalities in asymptomatic carriers.
Noticeably, most symptomatic patients of our study cohort were staged as PND1, representing the earlier disease phase, exclusively characterized by sensory and autonomic neuropathic symptoms [46]. This could explain why median sNfL levels in our patients appear to be lower than previously reported [21, 22] (Table 1).
As previously shown [13], a consistent proportion of these PND1 symptomatic subjects has normal NCS, making the instrumental demonstration of polyneuropathy onset challenging. In our study, the combination of sNfL (using a cut-off of 37.10 pg/mL) with distal IENFD and QST thermal thresholds (CDT and/or WDT), permitted to document neuropathic damage abnormalities in all symptomatic patients, in all cases in combination (e.g. elevated sNfL and reduced distal IENFD), thus remarking the potential usefulness of sNfL as a supportive criterion to identify symptomatic transition.
In our study sNfl levels significantly correlate with distal IENFD and the main small fiber related QST parameters, i.e. CDT and WDT. These correlations, corroborated by linear regression models, persisted even when the effect of the age was marginalized using age-adjusted normal values for distal-IENFD and z-score for QST parameters (Fig. 2). sNfl levels also correlated with sural SNAP; however, this correlation might be mostly driven by affected individuals, since in our study all the carriers had normal sural SNAP, as per selection criteria, whereas most symptomatic patients had abnormal sural SNAP.
Increasing knowledge suggests that sNfL levels are elevated in polyneuropathies at early stages [26]. A recent report showed sNfL levels increase even in patients with pure small fiber damage without large fiber neuropathy, leading the authors to propose sNfL as a surrogate biomarker for small fiber involvement detection in ATTRv with PN [47]. Conversely, other studies didn’t find any difference in sNfL levels between patients with small fiber neuropathy of various etiologies and healthy subjects, thus questioning the role of sNfL as a small fiber damage biomarker [48]. A plausible explanation for the inconsistency between findings in ATTRv-PN and small fiber neuropathies of other etiologies, may lie in the fact that small fiber neuropathy in ATTRv represents the detectable aspect of a broader spectrum of progressive damage, probably originating at the dorsal root ganglia level [14, 49, 50]. This hypothesis could elucidate why sNfL levels are elevated in ATTRv, even when patients exhibit symptoms solely attributable to small fiber neuropathy.
Considering that skin biopsy, the gold standard for small fiber assessment [43, 51], is not universally available, is time-demanding and minimally invasive, the elaboration of alternative, easier to perform biomarkers of small fiber damage is warranted and represents a compelling clinical challenge. Based on our results, we cannot assume that sNfL is a selective biomarker of small fiber degeneration in ATTRv-PN, since we found significant correlations between sNfL levels and large fiber mediated variables like sural SNAP amplitude. However, the correlations we found between sNfL and small fiber damage related parameters, which are early impaired in the disease course, confirm the potential usefulness of sNfL as a biomarker of nerve degeneration in ATTRv-PN.
Limitations
Even though small fiber damage assessment was performed according to widely agreed recommendations and only patients who underwent sNfL determination close to small fiber assessment were selected, the retrospective design may be considered as a limitation of our study. Further prospective, longitudinal, and follow up-validation studies are needed to confirm the potential yield of sNfL as a disease biomarker in ATTRv-PN. Another important limitation of our study is the relatively small sample size, since ATTRv-PN is a rare disease. However, we believe that our sample reliably reflects late-onset ATTRv-PN carriers’ and patients’ characteristics in our local population, due to age uniformity and strict selection criteria.
Admittedly, the clinical characteristics of our cohort, predominantly comprising patients with a mixed ATTRv phenotype, precluded us from drawing definitive conclusions regarding patients with prevalent cardiac involvement. Notably, we acknowledge the possibility that sNfL might not effectively identify the transition to symptomatic status in patients with dominant cardiac involvement, as evidenced by the normal sNfL levels observed in the three patients with a predominant cardiac phenotype in our study.
Moreover, the criteria utilized for distinguishing pre-symptomatic carriers from symptomatic patients may impact our findings regarding the efficacy of sNfL to distinguish between these groups. Specifically, we categorized patients as symptomatic if they reported at least two symptoms on the SFN-SIQ and had a NIS of ≥ 2, regardless of sural SNAP amplitude [13]. This approach could be viewed as more inclusive compared to previously published criteria [52]. However, we opted to include patients with normal sural SNAP in the symptomatic group only if they met the Besta criteria for definite small fiber neuropathy based on clinical, quantitative sensory testing (QST), and skin biopsy abnormalities [29].
Conclusions
Our study showed that sNfL dosage reliably discriminates symptomatic ATTRv-PN patients from pre-symptomatic carriers, and found significant relations between sNfL levels, skin biopsy, and QST small fiber related parameters, thus suggesting that sNfL might be a valuable biomarker of peripheral nerve involvement in ATTRv-PN and a supportive criterion to identify symptomatic disease transition.
Data availability
Data related to this study are available upon reasonable request.
Abbreviations
- ATTRv-PN:
-
Hereditary transthyretin amyloidosis with polyneuropathy
- CDT:
-
Cold detection threshold
- CPT:
-
Cold pain threshold
- DMTs:
-
Disease modifying therapies
- HPT:
-
Heat pain threshold
- IENFD:
-
Intraepidermal nerve fiber density
- MPT:
-
Mechanical pain threshold
- NIS:
-
Neuropathy Impairment Scale
- PND:
-
Polyneuropathy disability scoring system
- QST:
-
Quantitative sensory testing
- SFN-SIQ:
-
Small Fiber Neuropathy Symptoms Inventory Questionnaire
- SNAP:
-
Sensory nerve action potential
- SNfL:
-
Serum neurofilament light chain
- TTR:
-
Transthyretin
- WDT:
-
Warm detection threshold
References
Adams D, Koike H, Slama M, Coelho T (2019) Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol 15(7):387–404
Adams D, Polydefkis M, Gonzalez-Duarte A, Wixner J, Kristen AV, Schmidt HH, Berk JL, Losada Lopez IA, Dispenzieri A, Quan D, Conceicao IM, Slama MS, Gillmore JD, Kyriakides T, Ajroud-Driss S, Waddington-Cruz M, Mezei MM, Plante-Bordeneuve V, Attarian S, Mauricio E, Brannagan TH 3rd, Ueda M, Aldinc E, Wang JJ, White MT, Vest J, Berber E, Sweetser MT, Coelho T, OLESG Patisiran Global (2021) Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol 20(1):49–59
Coelho T, Maia LF, da Silva AM, Cruz MW, Plante-Bordeneuve V, Suhr OB, Conceicao I, Schmidt HH, Trigo P, Kelly JW, Labaudiniere R, Chan J, Packman J, Grogan DR (2013) Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol 260(11):2802–2814
Brannagan TH, Wang AK, Coelho T, Waddington Cruz M, Polydefkis MJ, Dyck PJ, Plante-Bordeneuve V, Berk JL, Barroso F, Merlini G, Conceicao I, Hughes SG, Kwoh J, Jung SW, Guthrie S, Pollock M, Benson MD, Gertz M, N-TO-LE Investigators (2020) Early data on long-term efficacy and safety of inotersen in patients with hereditary transthyretin amyloidosis: a 2-year update from the open-label extension of the NEURO-TTR trial. Eur J Neurol 27(8):1374–1381
Adams D, Tournev IL, Taylor MS, Coelho T, Plante-Bordeneuve V, Berk JL, Gonzalez-Duarte A, Gillmore JD, Low SC, Sekijima Y, Obici L, Chen C, Badri P, Arum SM, Vest J, Polydefkis M, Collaborators H-A (2023) Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid 30(1):1–9
Ando Y, Adams D, Benson MD, Berk JL, Plante-Bordeneuve V, Coelho T, Conceicao I, Ericzon BG, Obici L, Rapezzi C, Sekijima Y, Ueda M, Palladini G, Merlini G (2022) Guidelines and new directions in the therapy and monitoring of ATTRv amyloidosis. Amyloid 29(3):143–155
Yang NC, Lee MJ, Chao CC, Chuang YT, Lin WM, Chang MF, Hsieh PC, Kan HW, Lin YH, Yang CC, Chiu MJ, Liou HH, Hsieh ST (2010) Clinical presentations and skin denervation in amyloid neuropathy due to transthyretin Ala97Ser. Neurology 75(6):532–538
Masuda T, Ueda M, Suenaga G, Misumi Y, Tasaki M, Izaki A, Yanagisawa Y, Inoue Y, Motokawa H, Matsumoto S, Mizukami M, Arimura A, Deguchi T, Nishio Y, Yamashita T, Inomata Y, Obayashi K, Ando Y (2017) Early skin denervation in hereditary and iatrogenic transthyretin amyloid neuropathy. Neurology 88(23):2192–2197
Masuda T, Ueda M, Misumi Y, Nomura T, Inoue Y, Isoguchi A, Kanenawa K, Tasaki M, Yamashita T, Sonoda Y, Obayashi K, Ando Y (2019) Reduced intraepidermal nerve fibre density in patients with hereditary transthyretin amyloidosis. Amyloid 26(sup1):79–80
Ebenezer GJ, Liu Y, Judge DP, Cunningham K, Truelove S, Carter ND, Sebastian B, Byrnes K, Polydefkis M (2017) Cutaneous nerve biomarkers in transthyretin familial amyloid polyneuropathy. Ann Neurol 82(1):44–56
Chao CC, Hsueh HW, Kan HW, Liao CH, Jiang HH, Chiang H, Lin WM, Yeh TY, Lin YH, Cheng YY, Hsieh ST (2019) Skin nerve pathology: Biomarkers of premanifest and manifest amyloid neuropathy. Ann Neurol 85(4):560–573
Freeman R, Gonzalez-Duarte A, Barroso F, Campagnolo M, Rajan S, Garcia J, Kim JY, Wang N, Orellana L, Gibbons C (2022) Cutaneous amyloid is a biomarker in early ATTRv neuropathy and progresses across disease stages. Ann Clin Transl Neurol 9(9):1370–1383
Leonardi L, Adam C, Beaudonnet G, Beauvais D, Cauquil C, Not A, Morassi O, Benmalek A, Trassard O, Echaniz-Laguna A, Adams D, Labeyrie C (2022) Skin amyloid deposits and nerve fiber loss as markers of neuropathy onset and progression in hereditary transthyretin amyloidosis. Eur J Neurol 29(5):1477–1487
Leonardi L, Galosi E, Vanoli F, Fasolino A, Di Pietro G, Luigetti M, Sabatelli M, Fionda L, Garibaldi M, Alfieri G, Lauletta A, Morino S, Salvetti M, Truini A, Antonini G (2022) Skin biopsy and quantitative sensory assessment in an Italian cohort of ATTRv patients with polyneuropathy and asymptomatic carriers: possible evidence of early non-length dependent denervation. Neurol Sci 43(2):1359–1364
Tozza S, Severi D, Palumbo G, Provitera V, Ruggiero L, Dubbioso R, Iodice R, Nolano M, Manganelli F (2022) Quantitative sensory testing in Late-Onset ATTRv presymptomatic subjects: a single center experience. Biomedicines 10(11):2877
Conceicao I, de Castro I, Diaz A, Castro J (2022) Quantitative sensory testing: a good tool to identify subclinical neuropathy in ATTRV30M amyloidosis patients? Amyloid 30(2):239–243
Leonardi L, Costanzo R, Forcina F, Morino S, Antonini G, Salvetti M, Luigetti M, Romano A, Primiano G, Guglielmino V, Fionda L, Garibaldi M, Lauletta A, Rossini E, Tufano L, Ceccanti M, Esposito N, Falco P, di Pietro G, Truini A, Galosi E (2023) Quantitative sensory testing and skin biopsy findings in late-onset ATTRv presymptomatic carriers: Relationships with predicted time of disease onset (PADO). J Peripher Nerv Syst 28(3):390–397
England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N, Lewis RA, Low PA, Fisher MA, Herrmann DN, Howard JF, Lauria G, Miller RG, Polydefkis M, Sumner AJ, N American Academy of N American Association of M Electrodiagnostic M American Academy of Physical, Rehabilitation (2009) Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of laboratory and genetic testing (an evidence-based review) Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 1(1):5–13
Carroll AS, Razvi Y, O’Donnell L, Veleva E, Heslegrave A, Zetterberg H, Vucic S, Kiernan MC, Rossor AM, Gillmore JD, Reilly MM (2024) Serum neurofilament light chain in hereditary transthyretin amyloidosis: validation in real-life practice. Amyloid 1–10
Kapoor M, Foiani M, Heslegrave A, Zetterberg H, Lunn MP, Malaspina A, Gillmore JD, Rossor AM, Reilly MM (2019) Plasma neurofilament light chain concentration is increased and correlates with the severity of neuropathy in hereditary transthyretin amyloidosis. J Peripher Nerv Syst 24(4):314–319
Maia LF, Maceski A, Conceicao I, Obici L, Magalhaes R, Cortese A, Leppert D, Merlini G, Kuhle J, Saraiva MJ (2020) Plasma neurofilament light chain: an early biomarker for hereditary ATTR amyloid polyneuropathy. Amyloid 27(2):97–102
Louwsma J, Brunger AF, Bijzet J, Kroesen BJ, Roeloffzen WWH, Bischof A, Kuhle J, Drost G, Lange F, Kuks JBM, Gans ROB, Hazenberg BPC, Nienhuis HLA (2021) Neurofilament light chain, a biomarker for polyneuropathy in systemic amyloidosis. Amyloid 28(1):50–55
Ticau S, Sridharan GV, Tsour S, Cantley WL, Chan A, Gilbert JA, Erbe D, Aldinc E, Reilly MM, Adams D, Polydefkis M, Fitzgerald K, Vaishnaw A, Nioi P (2021) Neurofilament Light Chain as a Biomarker of Hereditary Transthyretin-Mediated Amyloidosis. Neurology 96(3):e412–e422
Luigetti M, Di Paolantonio A, Guglielmino V, Romano A, Rossi S, Sabino A, Servidei S, Sabatelli M, Primiano G (2022) Neurofilament light chain as a disease severity biomarker in ATTRv: data from a single-centre experience. Neurol Sci 43(4):2845–2848
Loser V, Benkert P, Vicino A, Lim Dubois Ferriere P, Kuntzer T, Pasquier J, Maceski A, Kuhle J, Theaudin M (2023) Serum neurofilament light chain as a reliable biomarker of hereditary transthyretin-related amyloidosis-A Swiss reference center experience. J Peripher Nerv Syst 28(1):86–97
Romano A, Primiano G, Antonini G, Ceccanti M, Fenu S, Forcina F, Gentile L, Inghilleri M, Leonardi L, Manganelli F, Obici L, Sabino A, Sciarrone MA, Tozza S, Vitali F, Luigetti M (2024) Serum neurofilament light chain: a promising early diagnostic biomarker for hereditary transthyretin amyloidosis? Eur J Neurol 31(1):e16070
Quan D, Obici L, Berk JL, Ando Y, Aldinc E, White MT, Adams D (2023) Impact of baseline polyneuropathy severity on patisiran treatment outcomes in the APOLLO trial. Amyloid 30(1):49–58
Galosi E, Falco P, Di Pietro G, Leone C, Esposito N, De Stefano G, Di Stefano G, Truini A (2022) The diagnostic accuracy of the small fiber neuropathy symptoms inventory questionnaire (SFN-SIQ) for identifying pure small fiber neuropathy. J Peripher Nerv Syst 27(4):283–290
Devigili G, Rinaldo S, Lombardi R, Cazzato D, Marchi M, Salvi E, Eleopra R, Lauria G (2019) Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain 142(12):3728–3736
Adams D, Ando Y, Beirão JM, Coelho T, Gertz MA, Gillmore JD, Hawkins PN, Lousada I, Suhr OB, Merlini G (2021) Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy. J Neurol 268(6):2109–2122
Suresh R, Grogan M, Maleszewski JJ, Pellikka PA, Hanna M, Dispenzieri A, Pereira NL (2014) Advanced cardiac amyloidosis associated with normal interventricular septal thickness: an uncommon presentation of infiltrative cardiomyopathy. J Am Soc Echocardiogr 27(4):440–447
Galosi E, Leonardi L, Falco P, Di Pietro G, Fasolino A, Esposito N, Leone C, Di Stefano G, Inghilleri M, Luigetti M, Giovanni A, Truini A (2022) Functional and morphometric assessment of small-fibre damage in late-onset hereditary transthyretin amyloidosis with polyneuropathy: the controversial relation between small-fibre-related symptoms and diagnostic test findings. Amyloid 1–8
Provitera V, Gibbons CH, Wendelschafer-Crabb G, Donadio V, Vitale DF, Stancanelli A, Caporaso G, Liguori R, Wang N, Santoro L, Kennedy WR, Nolano M (2016) A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol 23(2):333–338
Galosi E, Di Pietro G, La Cesa S, Di Stefano G, Leone C, Fasolino A, Di Lionardo A, Leonetti F, Buzzetti R, Mollica C, Cruccu G, Truini A (2021) Differential involvement of myelinated and unmyelinated nerve fibers in painful diabetic polyneuropathy. Muscle Nerve 63(1):68–74
Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B (2006) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 123(3):231–243
Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C (2010) Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain 151(3):598–605
Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede RD, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, Ziegler D (2013) Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 154(9):1807–1819
Falcão de Campos C, Conceição I (2023) Updated evaluation of the safety, efficacy and tolerability of tafamidis in the treatment of hereditary transthyretin amyloid polyneuropathy. Drug Healthc Patient Saf 15:51–62
Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90(8):870–881
Bischof A, Manigold T, Barro C, Heijnen I, Berger CT, Derfuss T, Kuhle J, Daikeler T (2018) Serum neurofilament light chain: a biomarker of neuronal injury in vasculitic neuropathy. Ann Rheum Dis 77(7):1093–1094
Altmann P, De Simoni D, Kaider A, Ludwig B, Rath J, Leutmezer F, Zimprich F, Hoeftberger R, Lunn MP, Heslegrave A, Berger T, Zetterberg H, Rommer PS (2020) Increased serum neurofilament light chain concentration indicates poor outcome in Guillain-Barré syndrome. J Neuroinflammation 17(1):86
Kim SH, Choi MK, Park NY, Hyun JW, Lee MY, Kim HJ, Jung SK, Cha Y (2020) Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci Rep 10(1):7995
Truini A, Aleksovska K, Anderson CC, Attal N, Baron R, Bennett DL, Bouhassira D, Cruccu G, Eisenberg E, Enax-Krumova E, Davis KD, Di Stefano G, Finnerup NB, Garcia-Larrea L, Hanafi I, Haroutounian S, Karlsson P, Rakusa M, Rice ASC, Sachau J, Smith BH, Sommer C, Tölle T, Valls-Solé J, Veluchamy A (2023) Joint European Academy of Neurology-European Pain Federation-Neuropathic Pain Special Interest Group of the International Association for the Study of Pain guidelines on neuropathic pain assessment. Eur J Neurol 30(8):2177–2196
Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Solé J (2010) European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 17(7): 903-e49
Romano A, Guglielmino V, Bisogni G, Di Paolantonio A, Truini A, Minnella AM, Sciarrone MA, Vitali F, Maceroni M, Galosi E, Sabatelli M, Luigetti M (2023) Early detection of nerve involvement in presymptomatic TTR mutation carriers: exploring potential markers of disease onset. Neurol Sci 45(4):1675–1684
Sekijima Y, Ueda M, Koike H, Misawa S, Ishii T, Ando Y (2018) Diagnosis and management of transthyretin familial amyloid polyneuropathy in Japan: red-flag symptom clusters and treatment algorithm. Orphanet J Rare Dis 13(1):6
González-Moreno J, Gragera-Martínez Á, Rodríguez A, Borrachero-Garro C, García-Garrido S, Barceló C, Manovel-Sánchez A, Ribot-Sansó MA, Ibargüen-González L, Gomila R, Muñoz-Beamud F, Losada-López I, Cisneros-Barroso E (2024) Biomarkers of axonal damage to favor early diagnosis in variant transthyretin amyloidosis (A-ATTRv). Sci Rep 14(1):581
Baka P, Steenken L, Escolano-Lozano F, Steffen F, Papagianni A, Sommer C, Pogatzki-Zahn E, Hirsch S, Protopapa M, Bittner S, Birklein F (2024) Studying serum neurofilament light chain levels as a potential new biomarker for small fiber neuropathy. Eur J Neurol e16192
Tozza S, Severi D, Spina E, Iovino A, Aruta F, Ruggiero L, Dubbioso R, Iodice R, Nolano M, Manganelli F (2021) The neuropathy in hereditary transthyretin amyloidosis: A narrative review. J Peripher Nerv Syst 26(2):155–159
Leonardi L, Di Pietro G, Di Pasquale A, Vanoli F, Fionda L, Garibaldi M, Galosi E, Alfieri G, Lauletta A, Morino S, Salvetti M, Truini A, Antonini G (2022) High-resolution ultrasound of peripheral nerves in late-onset hereditary transthyretin amyloidosis with polyneuropathy: similarities and differences with CIDP. Neurol Sci 43(5):3387–3394
Cazzato D, Lauria G (2017) Small fibre neuropathy. Curr Opin Neurol 30(5):490–499
Conceição I, Damy T, Romero M, Galán L, Attarian S, Luigetti M, Sadeh M, Sarafov S, Tournev I, Ueda M (2019) Early diagnosis of ATTR amyloidosis through targeted follow-up of identified carriers of TTR gene mutations. Amyloid 26(1):3–9
Acknowledgements
ALNYLAM Pharmaceuticals Inc. provided financial support for the present study as an Investigator-Initiated Research study to LL and GA. Moreover, this work has been supported by the Italian Ministry of Health Young Researcher Project Grant (GR-2021-12372306 to AR, GP, and LL) as a part of a wider project on biomarker research in ATTRv.
The present study was also supported by an Investigator-Initiated Research to “Fondazione Policlinico Universitario Agostino Gemelli IRCCS” from Pfizer Inc. Pfizer Inc. had no role in the study design, data analysis, and interpretation of the results.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
GA, LL and SM received speaker honoraria from SOBI and ALNYLAM and travel grants from SOBI, ALNYLAM and Akcea therapeutics. ML speaker honoraria, travel grants and consulting fees from SOBI, ALNYLAM and PFIZER. EG received speaker honoraria from ALNYLAM.
Ethical approval
This study was carried out according to the principles of the 1964 Declaration of Helsinki. Each enrolled subject provided a written informed consent, and all study data were obtained and elaborated in accordance with our institutional ethical committee regulations. The study protocol was approved by Sapienza University Ethical Committee (UNTTR-19–1, protocol number 275 SA_2019 19/12/2019) and Ethical Committee of Fondazione Policlinico Agostino Gemelli IRCSS (Prot. ID 4108).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galosi, E., Costanzo, R., Forcina, F. et al. Serum neurofilament light chain levels correlate with small fiber related parameters in patients with hereditary transthyretin amyloidosis with polyneuropathy (ATTRv-PN). Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07562-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07562-0