Abstract
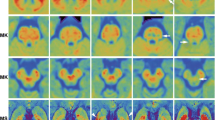
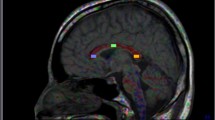
Paroxysmal kinesigenic choreoathetosis (PKC) is a rare neurologic disorder. There are not apparent morphological changes in patients with idiopathic PKC. The purpose of this study is to determine whether ultrastructural changes are in the brain of patients with idiopathic PKC using diffusion tensor imaging. From May 2007 to August 2008, seven patients with idiopathic PKC were included. The mean age at initial onset was 11.7 ± 3.1 (range 8–17) years, and the mean disease duration was 6.9 ± 5.1 (range 1–14) years. Seven subjects of an age- and sex-matched control group were recruited. DTI data were obtained with a 3-T scanner. Fractional anisotropy (FA) and mean diffusivity (MD) were obtained in eight brain regions of interest. Patients with idiopathic PKC had significantly higher FA values than controls in the right thalamus (P < 0.05 Bonferroni corrected). Patients also had lower MD values than controls in the left thalamus (P < 0.05 Bonferroni corrected). FA and MD values were not significantly correlated with age of onset, gender, frequency of attack and duration of the disease. The results showed that in patients with idiopathic PKC, diffusion tensor imaging discloses distinct ultrastructural abnormalities in the thalamus. DTI is a sensitive neuroradiologic technique for detecting cerebral alterations in patients even without visible lesions on conventional MRI.

Similar content being viewed by others
References
Bhatia KP (1999) The paroxysmal dyskinesias. J Neurol 246:149–155
Fahn S (1994) The paroxysmal dyskinesias. In: Marsden CD, Fahn S (eds) Movement disorders 3. Butterworth-Heinemann, Oxford, pp 310–345
Bennett LB, Roach ES, Bowcock AM (2000) A locus for paroxysmal kinesignic dyskinesia maps to human chromosome 16. Neurology 54:125–130
Ko CH, Kong CK, Ngai WT, Ma KM (2001) Ictal 99mTc ECD SPECT in paroxysmal kinesigenic choreoathetosis. Pediatr Neurol 24:225–227
Shirane S, Sasaki M, Kogure D, Matsuda H, Hashimoto T (2001) Increased ictal perfusion of the thalamus in paroxysmal kinesigenic dyskinesia. J Neurol Neurosurg Psychiatry 71:408–410
Volonte MA, Perani D, Lanzi R, Poggi A, Anchisi D, Balini A et al (2001) Regression of ventral striatum hypometabolism after calcium/calcitriol therapy in paroxysmal kinesigenic choreoathetosis due to idiopathic primary hypoparathyrodism. J Neurol Neurosurg Psychiatry 71:691–695
Pierpaoli C, Jezzard P, Blasser PJ, Barnett A, Di Chiro G (1996) Diffusion tensor MR imaging of the human brain. Radiology 201:637–648
Le Bihan D (2003) Looking into the functional architecture of the brain with diffusion MRI. Nature Rev Neurosci 4:469–480
Bealieu C (2002) The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 15:435–455
Fabbrinia G, Pantanoa P, Totaro P, Calistria V, Colosimoa C, Carmellinia M et al (2008) Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. Eur J Neurol 15:185–189
Chen Qin, Lui Su, Li Chun-Xiao, Jiang Li-Jun, Ou-Yang Luo, Tang He-Han et al (2008) MRI-negative refractory partial epilepsy: role for diffusion tensor imaging in high field MRI. Epilepsy Res 80:83–89
Chan LL, Rumpel H, Yap K, Lee E, Loo HV, Ho GL et al (2007) Case control study of diffusion tensor imaging in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78:1383–1386
Stefan K, Bogdan D, Charlotte VG, Carlton C, Zoltan N, Philip AC et al (2008) White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington’s disease. Brain 131:196–204
Kertesz A (1967) Paroxysmal kinesigenic choreoathetosis: an entity within the paroxysmal choreoathetosis syndrome. Description of 10 cases, including 1 autopsied. Neurology 17:680–690
Sunohara N, Mukoyama M, Mano Y, Satoyoshi E (1984) Action-induced rhythmic dystonia: an autopsy case. Neurology 34:321–327
Camac A, Greene P, Khandji A (1990) Paroxysmal kinesigenic dystonic choreoathetosis associated with a thalamic infarct. Mov Disord 5:235–238
Merchut MP, Brumlik J (1986) Painful tonic spasms caused by putaminal infarction. Stroke 17:1319–1321
Gilroy J (1982) Abnormal computed tomograms in paroxysmal kinesigenic choreoathetosis. Ann Neurol 39:779–780
Micheli F, Fernandez Pardal MM, Casas Parera I, Giannaula R (1986) Sporadic paroxysmal dystonic choreoathetosis associated with basal ganglia calcifications. Ann Neurol 20:750
Riley DE (1996) Paroxysmal kinesigenic dystonia associated with a medullary lesion. Mov Disord 11:738–740
Cosentino C, Torres L, Flores M, Cuba JM (1996) Paroxysmal kinesigenic dystonia and spinal cord lesion. Mov Disord 11:453–455
Melhem ER, Itoh R, Jones L, Barker PB (2000) Diffusion tensor MR imaging of the brain: effect of diffusion weighting on trace and anisotropy measurements. Am J Neuroradiol 21:1813–1820
Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM (2006) Diffusion tensor imaging in presymptomatic and early Huntington’s disease: selective white matter pathology and its relationship to clinical measures. Mov Disord 21:1317–1325
Harris JA, Guglielmotti V, Bentivoglio M (1996) Diencephalic asymmetries. Neurosci Biobehav Rev 20:637–643
Fabiano AJ, Horsfield MA, Bakshi R (2005) Interhemispheric asymmetry of brain diffusivity in normal individuals: a diffusion-weighted MR imaging study. Am J Neuroradiol 26:1089–1094
Ceballos-Baumann AO, Passingham RE, Marsden CD, Brooks DJ (1995) Motor reorganization in acquired hemidystonia. Ann Neurol 37:746–757
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos. 30625024 and 30728017) and Programs from the State Education Ministry (grant no. SRFDP-20060610073).
Conflict of interest statement
There was no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Zhou and Q. Chen contributed equally to this article.
Rights and permissions
About this article
Cite this article
Zhou, B., Chen, Q., Gong, Q. et al. The thalamic ultrastructural abnormalities in paroxysmal kinesigenic choreoathetosis: a diffusion tensor imaging study. J Neurol 257, 405–409 (2010). https://doi.org/10.1007/s00415-009-5334-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5334-9