Abstract
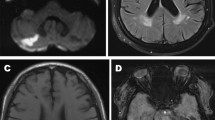
Fabry disease (FD) is a lysosomal storage disorder that is associated with marked cerebrovascular disease. Conventional MRI shows a progressive load of white matter lesions (WMLs) due to cerebral vasculopathy in the course of FD.
To quantify brain structural changes in clinically affected male and female patients with FD we performed a prospective Diffusion–Tensor Imaging (DTI) study in 27 adult Fabry patients (13m, 14f) and 21 age–matched controls (12 m, 9f).
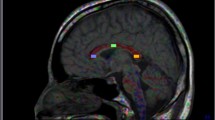
Global Mean Diffusivity (MD) was increased in FD (P = 0.003) whereas global Fractional Anisotropy (FA) did not differ significantly between FD and controls. Even FD patients without significant WMLs (9m, 9f) showed increased global MD (P = 0.004). Regions of interest with significant MD elevations were located in the frontal, parietal and temporal white matter. No differences of thalamic and hippocampal DTI measurements could be detected between FD and controls. DTI parameters did not differ between male and female patients.
The data provide the first evidence of a pattern of marked structural brain tissue alterations in adult FD male and female patients even without WMLs. DTI seems to be an appropriate diagnostic tool to quantify brain tissue integrity in FD. Moreover, this method could be favorable for longitudinal assessment of brain structure alterations in FD, and for monitoring the cerebral effects of enzyme replacement therapy.
Similar content being viewed by others
References
Meikle PJ, Hopwood JJ, Clague AE, Carey WF (1999) Prevalence of lysosomal storage disorders. JAMA 281:249–254
Davies JP, Eng CM, Hill JA, Malcolm S, MacDermot K, Winchester B, Desnick RJ (1996) Fabry disease: fourteen alpha–galactosidase A mutations in unrelated families from the United Kingdom and other European countries. Eur J Hum Genet 4:219–224
Mitsias P, Levine SR (1996) Cerebrovascular complications of Fabry’s disease. Ann Neurol 40:8–17
MacDermot KD, Holmes A, Miners AH (2001) Anderson–Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet 38:750–760
Mehta A, Ricci R, Widmer U, Dehout F, Garcia De Lorenzo A, Kampmann C, Linhart A, Sunder–Plassmann G, Ries M, Beck M (2004) Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest 34:236–242
Hilz MJ, Kolodny EH, Brys M, Stemper B, Haendl T, Marthol H (2004) Reduced cerebral blood flow velocity and impaired cerebral autoregulation in patients with Fabry disease. J Neurol 251:564–570
Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, Pease–Fye M, Ferri R, Brady RO, Herscovitch P, Schiffmann R (2001) Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation 104:1506–1512
Whybra C, Kampmann C, Willers I, Davies J, Winchester B, Kriegsmann J, Bruhl K, Gal A, Bunge S, Beck M (2001) Anderson–Fabry disease: clinical manifestations of disease in female heterozygotes. J Inherit Metab Dis 24:715–724
MacDermot KD, Holmes A, Miners AH (2001) Anderson–Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet 38:769–775
Crutchfield KE, Patronas NJ, Dambrosia JM, Frei KP, Banerjee TK, Barton NW, Schiffmann R (1998) Quantitative analysis of cerebral vasculopathy in patients with Fabry disease. Neurology 50:1746–1749
Moore DF, Herscovitch P, Schiffmann R (2001) Selective arterial distribution of cerebral hyperperfusion in Fabry disease. J Neuroimaging 11:303–307
Kolodny EH, Pastores GM (2002) Anderson–Fabry disease: extrarenal, neurologic manifestations. J Am Soc Nephrol 13(Suppl 2):S150–S153
Fellgiebel A MM, Mazanek M, Baron K, Beck M, Stoeter P (2005) White matter lesions severity in male and female patients with Fabry disease. Neurology (in press)
Pantoni L, Garcia JH (1997) Pathogenesis of leukoaraiosis: a review. Stroke 28:652–659
Prins ND, van Straaten EC, van Dijk EJ, Simoni M, van Schijndel RA, Vrooman HA, Koudstaal PJ, Scheltens P, Breteler MM, Barkhof F (2004) Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics. Neurology 62:1533–1539
Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13:534–546
Taylor WD, Hsu E, Krishnan KR, MacFall JR (2004) Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biol Psychiatry 55:201–207
Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T (2004) Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry 61:658–668
Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P (2004) Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord 18:101–108
Sach M, Winkler G, Glauche V, Liepert J, Heimbach B, Koch MA, Buchel C, Weiller C (2004) Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127:340–350
Pfefferbaum A, Sullivan EV (2002) Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage 15:708–718
Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR (2003) Fabry disease, an under–recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med 138:338–346
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self–diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254
O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS (2001) Normal–appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 57:2307–2310
Moore DF, Schiffmann R, Ulug AM (2002) Elevated CNS average diffusion constant in Fabry disease. Acta Paediatr Suppl 91:S67–S68
O’Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS (2004) Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 75:441–447
Fellgiebel A, Muller MJ, Mazanek M, Baron K, Beck M, Stoeter P (2005) White matter lesion severity in male and female patients with Fabry disease. Neurology 65:600–602
Barkhof F, Scheltens P (2002) Imaging of white matter lesions. Cerebrovasc Dis 13(Suppl 2):S21–S30
Chabriat H, Pappata S, Poupon C, Clark CA, Vahedi K, Poupon F, Mangin JF, Pachot–Clouard M, Jobert A, Le Bihan D, Bousser MG (1999) Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI. Stroke 30:2637–2643
Holtmannspotter M, Peters N, Opherk C, Martin D, Herzog J, Bruckmann H, Samann P, Gschwendtner A, Dichgans M (2005) Diffusion Magnetic Resonance Histograms as a Surrogate Marker and Predictor of Disease Progression in CADASIL. A Two–Year Follow–Up Study. Stroke
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fellgiebel, A., Mazanek, M., Whybra, C. et al. Pattern of microstructural brain tissue alterations in Fabry disease. J Neurol 253, 780–787 (2006). https://doi.org/10.1007/s00415-006-0118-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0118-y