Abstract.
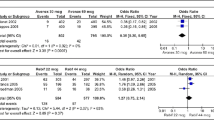
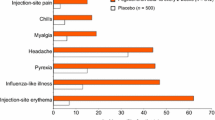
Protein-based therapies are useful in a variety of diseases; however, their potential for immunogenicity is a disadvantage. Neutralizing antibodies (NAbs) that develop to interferon beta (IFNβ) products (IFNβ-1b, IFNβ-1a-Avonex®, or IFNβ-1a-Rebif®), which are first-line therapies for the treatment of multiple sclerosis, are reported to reduce the clinical efficacy of these agents. In individual clinical studies of each commercially available IFNβ product, 28% to 47% of patients develop NAbs to IFNβ-1b, 12% to 28 % to IFNβ-1a-Rebif, and 2% to 6% to IFNβ-1a-Avonex. Problems exist in comparing the incidence of NAbs among IFNβ products across studies because of differences in study methodology, including assay methods, treatment duration, and the definition of NAb positive. Results from studies that have directly compared these products are consistent with results from the respective clinical trials of IFNβs. Both the clinical trials and the independent studies have shown that NAbs develop more frequently with IFNβ-1b treatment than with IFNβ-1a treatment and that, among IFNβ-1a products, NAbs develop more frequently with IFNβ-1a-Rebif treatment than with IFNβ-1a-Avonex treatment. Factors that may affect the immunogenicity of IFNβs, including the dosing regimens and the biochemical properties of the products, are discussed.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertolotto, A., Deisenhammer, F., Gallo, P. et al. Immunogenicity of interferon beta: differences among products. J Neurol 251 (Suppl 2), ii15–ii24 (2004). https://doi.org/10.1007/s00415-004-1204-7
Issue Date:
DOI: https://doi.org/10.1007/s00415-004-1204-7