Abstract
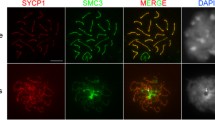
Pairing of the sex chromosomes during mammalian meiosis is characterized by the formation of a unique heterochromatin structure at the XY body. The mechanisms underlying the formation of this nuclear domain are reportedly highly conserved from marsupials to mammals. In this study, we demonstrate that in contrast to all eutherian species studied to date, partial synapsis of the heterologous sex chromosomes during pachytene stage in the horse is not associated with the formation of a typical macrochromatin domain at the XY body. While phosphorylated histone H2AX (γH2AX) and macroH2A1.2 are present as a diffuse signal over the entire macrochromatin domain in mouse pachytene spermatocytes, γH2AX, macroH2A1.2, and the cohesin subunit SMC3 are preferentially enriched at meiotic sex chromosome cores in equine spermatocytes. Moreover, although several histone modifications associated with this nuclear domain in the mouse such as H3K4me2 and ubH2A are conspicuously absent in the equine XY body, prominent RNA polymerase II foci persist at the sex chromosomes. Thus, the localization of key marker proteins and histone modifications associated with the XY body in the horse differs significantly from all other mammalian systems described. These results demonstrate that the epigenetic landscape and heterochromatinization of the equine XY body might be regulated by alternative mechanisms and that some features of XY body formation may be evolutionary divergent in the domestic horse. We propose equine spermatogenesis as a unique model system for the study of the regulatory networks leading to the epigenetic control of gene expression during XY body formation.








Similar content being viewed by others
References
Akhmedov A, Gross B, Jessberger R (1999) Mammalian smc3 c-terminal and coiled-coil protein domains specifically bind palindromic DNA, do not block DNA ends, and prevent DNA bending. J Biol Chem 274(53):38216–38224
Anderson SF, Schlegel BP, Nakajima T, Wolpin ES, Parvin JD (1998) BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat Genet 19:254–256
Baarends W, Hoogerbrugge J, Roest H, Ooms M, Vreeburg J, Hoeijmakers J, Grootegoed J (1999) Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol 207(2):322–333
Baarends W, Wassenaar E, van der Laan R, Hoogerbrugge J, Sleddens-Linkels E, Hoeijmakers J, de Boer P, Grootegoed J (2005) Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol 25(3):1041–1053
Bannister L, Schimenti J (2004) Homologous recombinational repair proteins in mouse meiosis. Cytogenet Genome Res 107(3–4):191–200
Burgoyne P, Mahadevaiah S, Turner J (2007) The management of DNA double-strand breaks in mitotic G(2), and in mammalian meiosis viewed from a mitotic G(2) perspective. Bioessays 29(10):974–986
Burgoyne P, Mahadevaiah S, Turner J (2009) The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 10(3):207–216
Chandley A, Jones R, Dott H, Allen W, Short R (1974) Meiosis in interspecific equine hybrids. I. The male mule (Equus asinus × E. caballus) and hinny (E. caballus × E. asinus). Cytogenet Cell Genet 13(4):330–341
De La Fuente R, Viveiros M, Burns K, Adashi E, Matzuk M, Eppig J (2004) Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev Biol 275(2):447–458
De La Fuente R, Baumann C, Fan T, Schmidtmann A, Dobrinski I, Muegge K (2006) Lsh is required for meiotic chromosome synapsis and retrotransposon silencing in female germ cells. Nat Cell Biol 8(12):1448–1454
de la Fuente R, Parra MT, Viera A, Calvente A, Gomez R, Suja JA, Rufas JS, Page J (2007) Meiotic pairing and segregation of achiasmate sex chromosomes in eutherian mammals: the role of SYCP3 protein. PLoS Genet 3(11):e198
Eijpe M, Heyting C, Gross B, Jessberger R (2000) Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes. J Cell Sci 113(Pt 4):673–682
Escalier D, Garchon H (2000) XMR is associated with the asynapsed segments of sex chromosomes in the XY body of mouse primary spermatocytes. Chromosoma 109(4):259–265
Fernandez-Capetillo O, Mahadevaiah S, Celeste A, Romanienko P, Camerini-Otero R, Bonner W, Manova K, Burgoyne P, Nussenzweig A (2003) H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell 4(4):497–508
Franco M, Sciurano R, Solari A (2007) Protein immunolocalization supports the presence of identical mechanisms of XY body formation in eutherians and marsupials. Chromosome Res 15(6):815–824
Froenicke L, Anderson L, Wienberg J, Ashley T (2002) Male mouse recombination maps for each autosome identified by chromosome painting. Am J Hum Genet 71(6):1353–1368
Handel MA (2004) The XY body: a specialized meiotic chromatin domain. Exp Cell Res 296(1):57–63
Handel M, Hunt P (1992) Sex-chromosome pairing and activity during mammalian meiosis. BioEssays 14(12):817–822
Hawley RS (2003) The human y chromosome: rumors of its death have been greatly exaggerated. Cell 113(7):825–828
Hoyer-Fender S (2003) Molecular aspects of XY body formation. Cytogenet Genome Res 103(3–4):245–255
Hoyer-Fender S, Singh P, Motzkus D (2000) The murine heterochromatin protein M31 is associated with the chromocenter in round spermatids and is a component of mature spermatozoa. Exp Cell Res 254(1):72–79
Hoyer-Fender S, Czirr E, Radde R, Turner J, Mahadevaiah S, Pehrson J, Burgoyne P (2004) Localisation of histone macroH2A1.2 to the XY-body is not a response to the presence of asynapsed chromosome axes. J Cell Sci 117(Pt 2):189–198
Jessberger R (2002) The many functions of SMC proteins in chromosome dynamics. Nat Rev Mol Cell Biol 3(10):767–778
Khalil AM, Driscoll DJ (2006) Histone H3 lysine 4 dimethylation is enriched on the inactive sex chromosomes in male meiosis but absent on the inactive X in female somatic cells. Cytogenet Genome Res 112(1–2):11–15
Khalil A, Driscoll D (2007) Trimethylation of histone H3 lysine 4 is an epigenetic mark at regions escaping mammalian X inactivation. Epigenetics 2(2):114–118
Khalil AM, Boyar FZ, Driscoll DJ (2004) Dynamic histone modifications mark sex chromosome inactivation and reactivation during mammalian spermatogenesis. Proc Natl Acad Sci USA 101(47):16583–16587
Losada A, Hirano T (2005) Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev 19(11):1269–1287
Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS (2001) Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet 27(3):271–276
Manders EEM, Verbeek FJ, Aten JA (1993) Measurement of co-localisation of objects in dual-colour confocal images. J Microsc 169:375–382
Monesi V (1965) Differential rate of ribonucleic acid synthesis in the autosomes and sex chromosomes during male meiosis in the mouse. Chromosoma 17(1):11–21
Namekawa SH, Lee JT (2009) XY and ZW: is meiotic sex chromosome inactivation the rule in evolution? PLoS Genet 5(5):e1000493
Namekawa S, VandeBerg J, McCarrey J, Lee J (2007) Sex chromosome silencing in the marsupial male germ line. Proc Natl Acad Sci USA 104(23):9730–9735
Page J, Berrios S, Rufas J, Parra M, Suja J, Heyting C, Fernandez-Donoso R (2003) The pairing of X and Y chromosomes during meiotic prophase in the marsupial species Thylamys elegans is maintained by a dense plate developed from their axial elements. J Cell Sci 116(3):551–560
Page J, de la Fuente R, Gómez R, Calvente A, Viera A, Parra M, Santos J, Berríos S, Fernández-Donoso R, Suja J, Rufas J (2006) Sex chromosomes, synapsis, and cohesins: a complex affair. Chromosoma 115(3):250–259
Pelttari J, Hoja M, Yuan L, Liu J, Brundell E, Moens P, Santucci-Darmanin S, Jessberger R, Barbero J, Heyting C, Hoog C (2001) A meiotic chromosomal core consisting of cohesin complex proteins recruits DNA recombination proteins and promotes synapsis in the absence of an axial element in mammalian meiotic cells. Mol Cell Biol 21(16):5667–5677
Perry J, Palmer S, Gabriel A, Ashworth A (2001) A short pseudoautosomal region in laboratory mice. Genome Res 11(11):1826–1832
Piras F, Nergadze S, Poletto V, Cerutti F, Ryder O, Leeb T, Raimondi E, Giulotto E (2009) Phylogeny of horse chromosome 5q in the genus Equus and centromere repositioning. Cytogenet Genome Res 126(1–2):165–172
Power M, Gustavsson I, Switonski M, Ploen L (1992) Synaptonemal complex analysis of an autosomal trisomy in the horse. Cytogenet Cell Genet 61(3):202–207
Prieto I, Tease C, Pezzi N, Buesa JM, Ortega S, Kremer L, Martinez A, Martinez AC, Hulten MA, Barbero JL (2004) Cohesin component dynamics during meiotic prophase I in mammalian oocytes. Chromosome Res 12(3):197–213
Revenkova E, Jessberger R (2006) Shaping meiotic prophase chromosomes: cohesins and synaptonemal complex proteins. Chromosoma 115(3):235–240
Richler C, Ast G, Goitein R, Wahrman J, Sperling R, Sperling J (1994) Splicing components are excluded from the transcriptionally inactive XY body in male meiotic nuclei. Mol Biol Cell 5(12):1341–1352
Scherthan H (2001) A bouquet makes ends meet. Nat Rev Mol Cell Biol 2(8):621–627
Schoenmakers S, Wassenaar E, Hoogerbrugge J, Laven J, Grootegoed J, Baarends W (2009) Female meiotic sex chromosome inactivation in chicken. PLoS Genet 5(5):e1000466
Sciurano R, Rahn M, Rey-Valzacchi G, Solari A (2007) The asynaptic chromatin in spermatocytes of translocation carriers contains the histone variant gamma-H2AX and associates with the XY body. Hum Reprod 22(1):142–150
Scott I, Long S (1980) An examination of chromosomes in the stallion (Equus caballus) during meiosis. Cytogenet Cell Genet 26(1):7–13
Solari A (1970a) The behaviour of chromosomal axes during diplotene in mouse spermatocytes. Chromosoma 31(2):217–230
Solari A (1970b) The spatial relationship of the X and Y chromosomes during meiotic prophase in mouse spermatocytes. Chromosoma 29(2):217–236
Tres LL (1977) Extensive pairing of the XY bivalent in mouse spermatocytes as visualized by whole-mount electron microscopy. J Cell Sci 25(1):1–15
Turner JM (2007) Meiotic sex chromosome inactivation. Development 134(10):1823–1831
Turner J, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli G, Barrett J, Burgoyne P, Deng C (2004) BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol 14(23):2135–2142
Turner J, Mahadevaiah S, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng C, Burgoyne P (2005) Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 37(1):41–47
Turner JM, Mahadevaiah SK, Ellis PJ, Mitchell MJ, Burgoyne PS (2006) Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev Cell 10(4):521–529
van der Heijden GW, Derijck AA, Posfai E, Giele M, Pelczar P, Ramos L, Wansink DG, van der Vlag J, Peters AH, de Boer P (2007) Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat Genet 39(2):251–258
Wade C, Giulotto E, Sigurdsson S, Zoli M, Gnerre S et al (2009) Genome sequence, comparative analysis, and population genetics of the domestic horse. Science 326(5954):865–867
White GE, Erickson HP (2006) Sequence divergence of coiled coils—structural rods, myosin filament packing, and the extraordinary conservation of cohesins. J Struct Biol 154(2):111–121
Wichman H, Payne C, Ryder O, Hamilton M, Maltbie M, Baker R (1991) Genomic distribution of heterochromatic sequences in equids: implications to rapid chromosomal evolution. J Hered 82(5):369–377
Xu X, Aprelikova O, Moens P, Deng C, Furth P (2003) Impaired meiotic DNA-damage repair and lack of crossing-over during spermatogenesis in BRCA1 full-length isoform deficient mice. Development 130(9):2001–2012
Zickler D (2006) From early homologue recognition to synaptonemal complex formation. Chromosoma 115(3):158–174
Zinchuk V, Zinchuk O (2008) Quantitative colocalization analysis of confocal fluorescence microscopy images. Curr Protoc Cell Biol 39:4.19.1–4.19.16
Zinchuk V, Zinchuk O, Okada T (2007) Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem Cytochem 40:101–111
Acknowledgments
We thank Dr. W. Earnshaw for the generous gift of human CREST antiserum and Drs. Michaela Kristula and Lauren Greene (Department of Clinical Studies, University of Pennsylvania) for providing equine testicular tissue. We are grateful to E. Amenkhienan for helping with preliminary data collection and to Dr. M.A. Handel for comments and critical reading of the manuscript. This research was supported by research grants from the University of Pennsylvania Research Foundation and the National Institutes of Health NIH 2RO1HDO42740 to R. De La Fuente. The support from McCabe Foundation (M. M. Viveiros) and the Havemeyer Foundation to S. M. McDonnell is also acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Keeney
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure S1
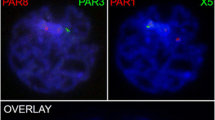
a Single channel images of the pachytene spermatocyte in Fig. 4c to illustrate the degree of signal overlap of SMC3 (green) and γH2AX (red) at chromosome cores (arrow and inset). b Immunochemical detection of RNA polymerase II (red) during meiotic progression in equine (upper panel) and mouse (lower panel) spermatocytes. Distinct RNA Pol II foci (arrow) were detected at the sex chromosome axes in the majority (>65%) of equine pachytene stage spermatocytes and in a small proportion (15%) of mouse spermatocytes (arrow). c Fluorescence in situ hybridization using an X chromosome-specific probe (red) reveals the extent of chromatin expansion (outlined) beyond sex chromosome cores (green) in equine pachytene spermatocytes. d RAD51 foci (red) associated with axial elements in fully synapsed mid-pachytene stage spermatocytes (lower cell, bold arrow) become resolved by the diplotene stage (upper cell) of meiotic prophase I. The sex chromosome bivalent shows a diffuse RAD51 signal with one to two foci associated with the PAR. DNA is shown in blue and SMC3 in green. Scale bar = 10 μm (GIF 424 kb)
Rights and permissions
About this article
Cite this article
Baumann, C., Daly, C.M., McDonnell, S.M. et al. Chromatin configuration and epigenetic landscape at the sex chromosome bivalent during equine spermatogenesis. Chromosoma 120, 227–244 (2011). https://doi.org/10.1007/s00412-010-0306-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-010-0306-5