Abstract
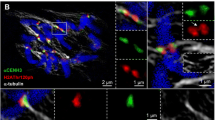
An atypical peg-like terminal constriction (“peg”) on metaphase chromosomes of the plant genus Oziroë could be identified as a nucleolus organizing region (NOR) by detecting 45S rDNA with correlative light microscopy (LM) and scanning electron microscopy (SEM) in situ hybridization (ISH). Using high-resolution 3D analytical SEM, the architecture and DNA distribution of the peg-like NOR were characterized as typical for chromosomes, albeit with significantly smaller chromomeres. ISH procedure was improved for SEM concerning signal localization, labeling efficiency, and structural preservation, allowing 3D SEM analysis of the peg-like NOR structure and rDNA distribution for the first time. It could be shown that implementation of FluoroNanogold markers is an attractive tool that allows efficient immunodection in both LM and SEM. A model is proposed for the peg structure and its mode of condensation.







Similar content being viewed by others
References
Allen TD, Jack EM, Harrison CJ (eds) (1988) The three dimensional structure of human metaphase chromosomes determined by scanning electron microscopy. Chromosomes and Chromatids. CRC, Florida
Berg C, Greilhuber J (1993) Cold-sensitive chromosome regions and heterochromatin in Cestrum aurantiacum (Solanaceae). Plant Syst Evol 185:259–273
Caperta AD, Neves N, Morais-Cecílo L, Malhó R, Viegas W (2002) Genome restructuring in rye affects the expression, organization and disposition of homologous rDNA loci. J Cell Sci 115(4):2839–2846
Castano IB, Brzoska PM, Sadoff BU, Chen H, Christman MF (1996) Mitotic chromosome condensation in the rDNA requires TRF4 and DNA topoisomerase I in Saccharomyces cerevisae. Genes Dev 10:2564–2576
Castilho A, Heslop-Harrison JS (1995) Physical mapping of 5S and 18S–25S rDNA and repetitive DNA sequences in Aegilops umbellulata. Genome 38:91–96
Chen Y, Zhao M, Li Z-P, He M-L (2002) The function of the nuclear matrix attachment region of silkworm rDNA as an autonomously replicating sequence in plasmid and chromsomal replication origin in yeast. Biochem Biophys Res Comm 299:723–729
Dobbs DL, Shaiu WL, Benbow RM (1994) Modular sequence elements associated with origin regions in eukaryotic chromosomal DNA. Nucleic Acids Res 22(13):2479–2489
Evans HJ, Buckland RA, Pardue ML (1974) Location of the genes coding for 18S and 28S ribosomal RNA in the human genome. Chromosoma 48:405–426
Freeman L, Aragon-Alcaide L, Stunnikov A (2000) The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J Cell Biol 149(4):811–824
Grau J (2000) “El Nino”—Leben für die untergehende Pflanzenweld der Atacama. Biol unserer Zeit 1:4–13
Guacci V, Hogan E, Koshland D (1994) Chromosome condensation and sister chromatid pairing in budding yeast. J Cell Biol 125:517–530
Guaglianone ER, Arroyo-Leuenberger S (2002) The South American genus Oziroë (Hyacinthaceae–Oziroeoideae). Darwiniana 40(1–4):61–76
Guttenbach M, Nassar N, Feichtinger W, Steinlein C, Nanda I, Wanner G, Kerem B, Schmid M (1998) An interstitial nucleoplus organizer region in the long arm of human chromosome 7: cytogenetic characterization and familial segregation. Cytogenet Cell Genet 80:104–112
Hainfeld JF, Powell RD (2000) New frontiers in gold labeling. J Histochem Cytochem 48(4):471–480
Henderson AS, Warburton D, Atwood KC (1972) Location of ribosomal DNA in the human chromosome complement. Proc Natl Acad Sci U S A 69(11):3394–3398
Houben A, Schubert I (2003) DNA and proteins of plant centromeres. Curr Opin Plant Biol 6:554–560
Howell WM, Black DA (1980) Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36:1014–1015
Jimenez R, Burgos M, Delaguardia RD (1988) A study of the Ag-staining significance in mitotic nors. Heredity 60:125–127
Lavoie BD, Hogan E, Koshland D (2004) In vivo requirements for rDNA chromosome condensation reveal two cell-cycle-regulated pathways for mitotic chromosome folding. Genes Dev 18:76–87
Lehfer H, Wanner G, Herrmann RG (1991) Physical mapping of DNA sequences on plant chromosomes by light microscopy and high resolution scanning electron microscopy. Plant Mol Biol 2:277–284
Long EO, Dawid IB (1980) Repeated genes in eukaryotes. Annu Rev Biochem 49:727–764
Martin R, Busch W, Herrmann RG, Wanner G (1994) Efficient preparation of plant chromosomes for high-resolution scanning electron microscopy. Chromosome Res 2:411–415
Martin R, Busch W, Herrmann RG, Wanner G (1995) In situ hybridization and signal detection by high resolution scanning electron microscopy. In: Brandham PE, Bennet MD (eds) Kew Chromosome Conference IV. Royal Botanic Gardens, Kew, England, pp 159–166
Nelson WG, Pienta KJ, Barrack ER, Coffey DS (1986) The role of the nuclear matrix in the organization and function of DNA. Ann Rev Biophys Biophys Chem 15:457–475
Neves N, Delgado M, Silva M, Caperta A, Morais-Cecílo L, Viegas W (2005) Ribosomal DNA heterochromatin in plants. Cytogenet Genome Res 109:104–111
Pikaard CS (2000) The epigenetics of nucleolar dominance. Trends Genet 16:495–500
Pontes O, Lawrence RJ, Neves N, Silva M, Lee J-H, Chen J, Viegas W, Pikaard CS (2003) Natural variation in nucleolar dominance reveals the relationship between nucleolus organizer chromatin topology and rRNA gene transcription in Arabidopsis. Proc Natl Acad Sci U S A 100(20):11418–11423
Raska I, Koberna K, Malinsky J, Fidlerova H, Masata M (2004) The nucleolus and transcription of ribosomal genes. Biol Cell 96:579–594
Savage JRK (2004) On the nature of visible chromosomal gaps and breaks. Cytogenet Genome Res 104:46–55
Schroeder-Reiter E, Houben A, Wanner G (2003) Immunogold labeling of chromosomes for scanning electron microscopy: a closer look at phosphorylated histone H3 in mitotic metaphase chromosomes of Hordeum vulgare. Chromosome Res 11:585–596
Schubert I (1984) Mobile nucleolus organizing regions (NORs) in allium (Liliaceae S-Lat)—inferences from the specificity of silver staining. Plant Syst Evol 144:291–305
Schweizer D (1973) Differential staining of plant chromosomes with Giemsa. Chromosoma 40:307–320
Speta F (1998) Systematische Analyse der Gattung Scilla L. s.l. (Hyacinthaceae). Phyton (Horn) 38:1–141
Sumner AT (1991) Scanning electron microscopy of mammalian chromosomes from prophase to telophase. Chromosoma 100:410–418
Wanner G, Formanek H (1995) Imaging of DNA in human and plant chromosomes by high-resolution scanning electron microscopy. Chromosome Res 3(6):368–374
Wanner G, Formanek H (2000) A new chromosome model. J Struct Biol 132:147–161
Wanner G, Schroeder-Reiter E, Formanek H (2005) 3D analysis of chromosome architecture: advantages and limitations with SEM. Cytogenet Genome Res 109:70–78
Yakura K, Tanifuji S (1983) Molecular cloning and restriction analysis of EcoRI-fragments of Vicia faba rDNA. Plant Cell Physiol 24:1327–1330
Zoller JF (2003) Hochauflösende Strukturanalyse pflanzlicher Chromosomen in Mitose und Meiose. Dissertation der Fakultät für Biologie. Ludwig-Maximilians-Universität München, Munich, Germany (248 pages)
Acknowledgements
The authors thank Katrin Kumke, Sabine Steiner, and Emilie Vosyka for excellent technical assistance, Dr. Helmut Formanek for supplying the Pt blue compound and for support in researching molecular sizes, and Renate Reichinger-Bock for artwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Nigg
Rights and permissions
About this article
Cite this article
Schroeder-Reiter, E., Houben, A., Grau, J. et al. Characterization of a peg-like terminal NOR structure with light microscopy and high-resolution scanning electron microscopy. Chromosoma 115, 50–59 (2006). https://doi.org/10.1007/s00412-005-0030-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-005-0030-8