Abstract
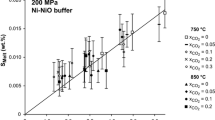
Hydrothermal volatile-solubility and partitioning experiments were conducted with fluid-saturated haplogranitic melt, H2O, CO2, and S in an internally heated pressure vessel at 900°C and 200 MPa; three additional experiments were conducted with iron-bearing melt. The run-product glasses were analyzed by electron microprobe, FTIR, and SIMS; and they contain ≤0.12 wt% S, ≤0.097 wt% CO2, and ≤6.4 wt% H2O. Apparent values of log f O2 for the experiments at run conditions were computed from the [(S6+)/(S6++S2−)] ratio of the glasses, and they range from NNO −0.4 to NNO + 1.4. The C–O–H–S fluid compositions at run conditions were computed by mass balance, and they contained 22–99 mol% H2O, 0–78 mol% CO2, 0–12 mol% S, and <3 wt% alkalis. Eight S-free experiments were conducted to determine the H2O and CO2 concentrations of melt and fluid compositions and to compare them with prior experimental results for C–O–H fluid-saturated rhyolite melt, and the agreement is excellent. Sulfur partitions very strongly in favor of fluid in all experiments, and the presence of S modifies the fluid compositions, and hence, the CO2 solubilities in coexisting felsic melt. The square of the mole fraction of H2O in melt increases in a linear fashion, from 0.05 to 0.25, with the H2O concentration of the fluid. The mole fraction of CO2 in melt increases linearly, from 0.0003 to 0.0045, with the CO2 concentration of C–O–H–S fluids. Interestingly, the CO2 concentration in melts, involving relatively reduced runs (log f O2 ≤ NNO + 0.3) that contain 2.5–7 mol% S in the fluid, decreases significantly with increasing S in the system. This response to the changing fluid composition causes the H2O and CO2 solubility curve for C–O–H–S fluid-saturated haplogranitic melts at 200 MPa to shift to values near that modeled for C–O–H fluid-saturated, S-free rhyolite melt at 150 MPa. The concentration of S in haplogranitic melt increases in a linear fashion with increasing S in C–O–H–S fluids, but these data show significant dispersion that likely reflects the strong influence of f O2 on S speciation in melt and fluid. Importantly, the partitioning of S between fluid and melt does not vary with the (H2O/H2O + CO2) ratio of the fluid. The fluid-melt partition coefficients for H2O, CO2, and S and the atomic (C/S) ratios of the run-product fluids are virtually identical to thermodynamic constraints on volatile partitioning and the H, S, and C contents of pre-eruptive magmatic fluids and volcanic gases for subduction-related magmatic systems thus confirming our experiments are relevant to natural eruptive systems.








Similar content being viewed by others
References
Aranovich LY, Newton RC (1999) Experimental determination of CO2–H2O activity-composition relations at 600–1000°C and 6–14 kbar by reversed decarbonation and dehydration reactions. Am Miner 84:1319–1332
Beermann O (2010) The solubility of sulfur and chlorine in H2O-bearing dacites of Krakatau and basalts of Mt. Etna. Dissertation, University of Hannover, 109 p
Behrens H, Tamic N, Holtz F (2004) Determination of the molar absorption coefficient for the infrared absorption band of CO2 in rhyolitic glasses. Am Miner 89:301–306
Berkesi M, Hidas K, Guzmics T, Dubessy J, Bodnar RJ, Szabo C, Vajna B, Tsunogae T (2009) Detection of small amounts of H2O in CO2-rich fluid inclusions using Raman spectroscopy. J Raman Spectrosc 40:1461–1463
Blank JG, Stolper EM, Carroll MR (1993) Solubilities of carbon dioxide and water in rhyolitic melt at 850°C and 750 bars. Earth Planet Sci Lett 119:27–36
Botcharnikov RE, Behrens H, Holtz F, Koepke J, Sato H (2004) Sulfur and chlorine solubility in Mt. Unzen rhyodacite melt at 850°C and 200 MPa. Chem Geol 213:207–225
Botcharnikov RE, Freise M, Holtz F, Behrens H (2005) Solubility of C-O-H mixtures in natural melts: new experimental data and application range of recent models. Ann Geophys 48:633–646
Burgisser A, Scaillet B (2007) Redox evolution of a degassing magma rising to the surface. Nature 445:194–197
Burgisser A, Scaillet B, Harshvardhan (2008) Chemical patterns of erupting silicic magmas and their influence on the amount of degassing during ascent. J Geophys Res 113(B12204):14
Carroll MR, Rutherford MJ (1988) Sulfur speciation in hydrous experimental glasses of varying oxidation state: results from measured wavelength shifts of sulfur X-rays. Am Min 73:845–849
Carroll MR, Webster JD (1994) Solubilities of sulfur, noble gases, nitrogen, chlorine, and fluorine in magmas. Rev Miner 30:231–279
Cioni R, Marianelli P, Santacroce R, Sbrana A (2000) Plinian and subplinian eruptions. In: Sigurdsson H (ed) Encyclopedia of volcanoes. Academic Press, San Diego, pp 477–494
Clemente B, Scaillet B, Pichavant M (2004) The solubility of sulphur in hydrous rhyolitic melts. J Petrol 45:2171–2196
Fogel RA, Rutherford MJ (1990) The solubility of carbon dioxide in rhyolitic melts: a quantitative FTIR study. Am Miner 75:1311–1326
Gladstone JH, Dale TP (1864) Researches on the refraction, dispersion, and sensitiveness of liquids. Phil Trans R Soc London 153:317–343
Gorbachev NS (1990) Fluid-magma interaction in sulfide-silicate systems. Int Geol Rev 32:749–836
Holloway JR (1987) Igneous fluids. Rev Miner 17:211–232
Holloway JR, Blank J (1994) Application of experimental results to C-O-H species in natural melts. Rev Miner 30:187–230
Jugo P, Wilke M, Botcharnikov RE (2010) Sulfur K-edge XANES analysis of natural and synthetic basaltic glasses: implications for S speciation and S content as function of oxygen fugacity. Geochim Cosmochim Acta 74:5926–5938
Keppler H (1999) Experimental evidence for the source of excess sulfur in explosive volcanic eruptions. Science 284:1652–1654
Keppler H (2010) The distribution of sulfur between haplogranite melts and aqueous fluids. Geochim Cosmochim Acta 74:645–660
Kerrick DM, Jacobs GK (1981) A modified Redlich-Kwong equation for H2O, CO2, and H2O-CO2 mixtures at elevated pressures and temperatures. Am J Sci 281:735–767
Lesne P, Kohn SC, Blundy J, Witham F, Botcharnikov RE, Behrens H (in press) Experimental simulation of basalt degassing at Stromboli and Masaya volcanoes. J Petrol
Lin B, Bodnar RJ (2010) Synthetic fluid inclusions XVIII: experimental determination of the PVTX properties of H2O-CH4 to 500°C, 3 kbar and XCH4 ≤ 4 mol%. Geochim Cosmochim Acta 74:3260–3273
Liu Y, Zhang Y, Behrens H (2005) Solubility of H2O in rhyolitic melts at low pressures and a new empirical model for mixed H2O-CO2 solubility in rhyolitic melts. J Volcanol Geotherm Res 143:235–291
Mandeville CW, Webster JD, Rutherford MJ, Taylor BE, Timbal A, Faure K (2002) Determination of molar aborptivities for infrared absorption bands of H2O in andesitic glasses. Am Min 87:813–821
Matthews SJ, Moncrieff DHS, Carroll MR (1999) Empirical calibration of the sulphur valence oxygen barometer from natural and experimental glasses; method and applications. Miner Mag 63(3):421–431
Métrich N, Clocchiatti R (1996) Sulfur abundance and its speciation in oxidized alkaline melts. Geochim Cosmochim Acta 60:4151–4160
Métrich N, Wallace PJ (2008) Volatile abundances in basaltic magmas and their degassing paths tracked by melt inclusions. Rev Mineral Geochem 69:363–402
Moore G (2008) Interpreting H2O and CO2 contents in melt inclusions: constraints from solubility experiments and modeling. Rev Mineral Geochem 69:333–361
Moretti R, Baker DR (2008) Modeling the interplay of f O2 and f S2 along the FeS-silicate melt equilibrium. Chem Geol 256:286–298
Moretti R, Papale P (2004) On the oxidation state and volatile behavior in multicomponent gas-melt equilibria. Chem Geol 213:265–280
Morizet Y, Paris M, Gaillard F, Scaillet B (2010) C-O-H fluid solubility in haplobasalt under reducing conditions: an experimental study. Chem Geol 279:1–16
Mysen BO, Popp RK (1980) Solubility of sulfur in CaMgSi2O6 and NaAlSi3O8 melts at high-pressure and temperature with controlled f O2 and f S2. Am J Sci 280(1):78–92
Newman S, Lowenstern JB (2002) VOLATILECALC: a silicate melt-H2O-CO2 solution model written in Visual Basic for excel. Comp Geosci 28:597–604
Nicholis MG, Rutherford MJ (2009) Graphite oxidation in the Apollo 17 orange glass magma: implications for the generation of a lunar volcanic gas phase. Geochim Cosmochim Acta 73:5905–5917
Nowak M, Behrens H (1995) The speciation of water in haplogranitic glasses and melts determined by in situ near-infrared spectroscopy. Geochim Cosmochim Acta 59:3445–3450
Nowak M, Behrens H (1997) An experimental investigation of diffusion of water in haplogranitic melts. Contrib Mineral Petrol 126:365–376
Oppenheimer C (2003) Volcanic degassing. In: Rudnick RL (ed) The crust. Treatise in geochemistry, vol 3. Elsevier, Amsterdam, pp 123–166
Parat F, Holtz F, Feig S (2008) Pre-eruptive conditions of the Huerto Andesite (Fish Canyon System, San Juan volcanic field, Colorado): influence of volatiles (C-O-H-S) on phase equilibria and mineral composition. J Petrol 49:911–935
Ripley E, Li C, Moore CH, Elswick K, Maynard JB, Paul RL, Sylvester P, Seo JH, Shimizu N (in press) Analytical methods for sulfur determination in glasses, rocks, minerals and fluid inclusions. In: Behrens H, Webster JD (eds) Sulfur in magmas and melts and its importance for natural and technical processes, vol 74. Rev Mineral Geochem
Scaillet B, Pichavant M (2003) Experimental constraints on volatile abundances in arc magmas and their implications for degassing processes. In: Oppenheimer C, Pyle DM, Barclay J (eds) Volcanic degassing, vol 213. Geol Soc Spec Pub, pp 23–52
Scaillet B, Pichavant M (2005) A model of sulphur solubility for hydrous mafic melts: application to the determination of magmatic fluid compositions of Italian volcanoes. Ann Geophys 48(4–5):671–698
Scaillet B, Clemente B, Evans BW, Pichavant M (1998) Redox control of sulfur degassing in silicic magmas. J Geophys Res 103(B10):23937–23949
Scaillet B, Luhr JF, Carroll MR (2003) Petrological and volcanological constraints on volcanic sulfur emissions to the atmosphere. AGU Geophys Mono 139:11–40
Silver LA, Ihinger PD, Stolper E (1990) The influence of bulk composition on the speciation of water in silicate glasses. Contrib Mineral Petrol 104:142–162
Spilliaert N, Metrich N, Allard P (2006) S-Cl-F degassing pattern of water-rich alkali basalt: Modelling and relationship with eruption styles on Mount Etna volcano. Earth Planet Sci Lett 248(3–4):772–786
Tamic N, Behrens H, Holtz R (2001) The solubility of H2O and CO2 in rhyolitic melts in equilibrium with a mixed CO2–H2O fluid phase. Chem Geol 174:333–347
Teague AJ, Kohn SC, Klimm K, Botcharnikov RE (2008) Sulphur solubility in Mount Hood andesites and CO2 fluids: implications for volcanic degassing EOS Trans. AGU, 89(53), (Fall Meet. Suppl. Abstract V21B-2086)
Wallace PJ, Carmichael ISE (1994) S speciation in submarine basaltic glasses as determined by measurements of SKα X-ray wavelength shifts. Am Miner 79:161–167
Wallace PJ, Edmonds M (in press) The sulfur budget in magmas: evidence from melt inclusions, submarine glasses, and volcanic gas emissions. In: Behrens H, Webster JD (eds) Sulfur in magmas and melts and its importance for natural and technical processes, vol 74. Rev Min Geochem
Webster JD (1992a) Fluid-melt interactions involving Cl-rich granites: experimental study from 2 to 8 kbar. Geochim Cosmochim Acta 56:659–678
Webster JD (1992b) Water solubility and chlorine partitioning in Cl-rich granitic systems: effects of melt composition at 2 kbar and 800°C. Geochim Cosmochim Acta 56:679–687
Webster JD, Botcharnikov RE (in press) Distribution of sulfur between melt and fluid in S-O-H-C-Cl-bearing magmatic systems at shallow crustal P-T conditions. In: Behrens H, Webster JD (eds) Sulfur in magmas and melts and its importance for natural and technical processes, vol 74. Rev Min Geochem
Webster JD, Sintoni MF, De Vivo B (2006) The role of sulfur in promoting magmatic degassing and volcanic eruption at Mt. Somma-Vesuvius. In: De Vivo B (ed) Volcanism in the Campania plain, vol 9. Elsevier, Amsterdam, pp 219–233
Webster JD, Sintoni MF, De Vivo B (2009) The partitioning behavior of Cl and S in aqueous fluid- and saline-liquid saturated phonolitic and trachytic melts at 200 MPa. Chem Geol 263:19–36
Acknowledgments
We appreciate thoughtful reviews by Editor Tim Grove and two anonymous referees. We also wish to recognize discussions on analytical methods with Charles Mandeville. Our thanks are offered to Professor David London of the University of Oklahoma who provided the haplogranite starting glass and Professor Harald Behrens of the University of Hannover, Germany, who provided two synthetic glasses with measured H2O contents that were used to test our FTIR analyses. Robert Bodnar and Charles Farley, of Virginia Tech, kindly conducted Raman analyses of 9 of the run-product glasses to determine the dominant volatile species in the vesicles. This research was supported in part by National Science Foundation awards EAR 0308866 and EAR-0836741 to J.D.W.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. L. Grove.
Rights and permissions
About this article
Cite this article
Webster, J.D., Goldoff, B. & Shimizu, N. C–O–H–S fluids and granitic magma: how S partitions and modifies CO2 concentrations of fluid-saturated felsic melt at 200 MPa. Contrib Mineral Petrol 162, 849–865 (2011). https://doi.org/10.1007/s00410-011-0628-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00410-011-0628-1