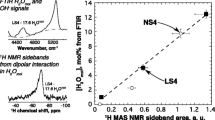
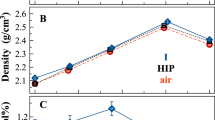
Abstract
Infrared spectroscopy was used to determine the concentrations of molecular water and hydroxyl groups in hydrous rhyolitic, orthoclasic, jadeitic, and Ca−Al-silicate glasses synthesized by quenching of melts from elevated presure and temperature. The rhyolitic glasses and some of the Ca−Al-silicate glasses were quenched from water-vapor-saturated melts and used to determine the solubility of water in melts of these compositions. For all compositions studied, hydroxyl groups are the dominant hydrous species at low total water contents, whereas molecular water dominates at elevated water contents. Although the trends in species concentrations in all these compositions are similar, the proportions of the two hydrous species are influenced by silicate chemistry: increasing silica content and K relative to Na both favor molecular water over hydroxyl. Results on rhyolitic glass demonstrate that molecular water is also favored by decreasing temperature at T<850°C. For rhyolitic glasses quenched from vapor-saturated melts, the mole fraction of molecular water is proportional to water fugacity for P(H2O)≤1500 bars, demonstrating that the behavior of molecular water is approximately Henrian at total water contents up to at least several weight percent. Data on water solubility for albitic, orthoclasic, and Ca−Al-silicate melts to higher pressures can also be fit by assuming Henrian behavior for molecular water and can be used to set constraints on the partial molar volume of water in these melts. The demonstration of Henry's law for molecular water in these liquids provides a link between spectroscopic measurements of microscopic species concentrations and macroscopic thermodynamic properties.
Similar content being viewed by others
References
Acocella J, Tomozawa M, Watson EB (1984) The nature of dissolved water in sodium silicate glasses and its effect on various properties. J Non-Cryst Solids 65:175–183
Aines RD, Silver LA, Rossman BR, Stolper EM, Holloway JR (1984) Direct observation of water speciation in rhyolite at temperatures up to 850°C (abs). In: Geol Soc Am 96th Ann Mtg 15(6):512
Albarede F, Provost A (1977) Petrological and geochemical mass-balance equations: an algorithm for least-squares fitting and general error analysis. Comput Geosci 3:309–326
Arndt J, Haberle F (1973) Thermal expansion and glass transition temperatures of synthetic glasses of plagioclase-like compositions. Contrib Mineral Petrol 39:175–183
Bartholomew RF, Schreurs IWH (1980) Wide-line NMR study of the protons in hydrosilicate glasses of different water content. J Non-Cryst Solids 38/39:679–684
Bartholomew RF, Butler BL, Hoover HL, Wu CK (1980) Infrared spectra of a water-containing glass. J Am Ceram Soc 63:481–485
Boulos EN, Kreidl NJ (1972) Water in glass: a review. J Canad Ceram Soc 41:83–90
Burnham CW (1975) Water and magmas: a mixing model. Geochim Cosmochim Acta 39:1077–1084
Burnham CW (1979) The importance of volatile constituents. In: Yoder HS Jr (ed) The evolution of the igneous rocks. Princeton Univ Press, Princeton, pp 439–482
Burnham CW, Davis NF (1971) The role of H2O in silicate melts: I. P-V-T relations in the system NaAlSi3O8−H2O to 1 kilobars and 1000° C. Am J Sci 270:54–79
Burnham CW, Jahns RH (1962) A method for determining the solubility of water in silicate melts. Am J Sci 260:721–745
Eckert H, Yesinowski JP, Stolper EM, Stanton TR, Holloway J (1987) The state of water in rhyolitic glasses: a deuterium NMR study. J Non-Cryst Solids 93:93–114
Eckert H, Yesinowski JP, Silver LA, Stolper EM (1988) Water in silicate glasses: quantitation and structural studies by 1H solid echo and MAS-NMR methods. J Chem Phys 92:2055–2064
Epel'baum MB (1985) The structure and properties of hydrous granitic melts. Geol Carpath 36:491–498
Ernsberger FM (1977) Molecular water in glass. J Am Ceram Soc 60:91
Farnan I, Kohn SC, Dupree R (1987) A study of the structural role of water in hydrous silica glass using cross-polarization magic angle spinning NMR. Geochim Cosmochim Acta 51:2869–2873
Gladstone JH, Dale TP (1864) Researches on the refraction, dispersion, and sensitiveness of liquids. Philos Trans R Soc London 153:317–343
Hamilton DL, Oxtoby S (1986) Solubility of water in albite-melt determined by the weight-loss method. J Geol 94:626–630
Holloway JR (1977) Fugacity and activity of molecular species in supercritical fluids. In: Fraser D (ed) Thermodynamics in geology. Reidel, Boston, pp 161–181
Johannes W, Bell PM, Mao HK, Boettcher AL, Chipman DW, Hays JF, Newton RC, Seifert F (1971) An interlaboratory comparison of piston-cylinder calibration using the albite-break-down reaction. Contrib Mineral Petrol 32:24–38
Karsten JL, Holloway JR, Delaney JR (1982) Ion microprobe studies of water in silicate melts: temperature-dependent water diffusion in obsidian. Earth Planet Sci Lett 59:420–428
Kurkjian CR, Russell LE (1958) Solubility of water in molten alkali silicates. J Soc Glass Techn 42:130T-144T
Kushiro I (1978) Viscosity and structural changes of albite (NaAlSi3O8) melt at high pressures. Earth Planet Sci Lett 41:87–90
Langer K, Flörke OW (1974) Near infrared absorption spectra (4000–9000 cm-1) of opals and the role of “water” in these SiO2·nH2O minerals. Fortsch Mineral 52:17–51
Larsen ES (1909) The relationship between refractive index and density of some crystallized silicates and their glasses. Am J Sci 28:263–274
McMillan PF, Holloway JR (1987) Water solubility in aluminosilicate melts. Contrib Mineral Petrol 97:320–332
McMillan PF, Jakobsson S, Holloway JR, Silver LA (1983) A note on the Raman spectra of water-bearing albite glass. Geochim Cosmochim Acta 47:1937–1943
McMillan PF, Peraudeau G, Holloway J, Coutures J-P (1986) Water solubility in a calcium aluminosilicate melt. Contrib Mineral Petrol 94:178–182
McMillan PW, Chlebik A (1980) The effect of hydroxyl ion content on the mechanical and other properties of soda-lime-silica glass. J Non-Cryst Solids 38/39:509–514
Morey GW (1954) The properties of glass (2nd ed). ACS Monograph 124. Reinhold, New York, p 591
Moulson AJ, Roberts JP (1960) Water in silica glass. Trans Brit Ceram Soc 59:388–399
Mysen BO, Virgo D (1986a) Volatiles in silicate melts at high pressure and temperature. 1. Interaction between OH groups and Si4+, Al3+, Ca2+, Na+ and H+. Chem Geol 57:303–331
Mysen BO, Virgo D (1986b) Volatiles in silicate melts at high pressure and temperature. 2. Water in melts along the join NaAlO2−SiO2 and a comparison of solubility mechanisms of water and fluorine. Chem Geol 57:333–358
Newman S, Stolper EM, Epstein S (1986) Measurement of water in rhyolitic glasses: Calibration of an infrared spectroscopic technique. Am Mineral 71:1527–1541
Newman S, Epstein S, Stolper EM (1988) Water, carbon dioxide, and hydrogen isotopes in glasses from the ca. 1340 A.D. eruption of the Mono Craters, California: constraints on degassing phenomena and initial volatile content. J Volcanol Geotherm Res 35:75–96
Orlova GP (1962) The solubility of water in albite melts. Int Geol Rev 6:254–258
Ostrovskiy IA, Orlova GP, Rudnitskaya YS (1964) Stoichiometry in the solution of water in alkali-aluminosilicate melts. Doklady Akad Nauk SSR 157:149–151
Oxtoby S, Hamilton DL (1978) Solubility of water in melts of the Na2O−Al2O3−SiO2 and K2O−Al2O3−SiO2 systems. In: Mackenzie WS (ed) Progress in experimental petrology. Natl Environ Res Council Pub Ser D No 11, Dept Geology, Manchester Univ
Persikov ES (1972) Experimental studies of solubility of water in granitic melt and kinetics of the melt-water equilibria at high pressures. Int Geol Rev 16:1062–1067
Pichavant M, Ramboz C (1985) Première détermination expérimentale des relations de phases dans le système haplogranitique en conditions de sous-saturation en H2O. CR Acad Sci Paris 301, Ser II:607–610
Pickett DA, Stolper EM (1984) Thermometry of rhyolitic obsidians based on water speciation (abs). EOS Trans Am Geophys Un 65:1128
Shaw HR (1964) Theoretical solubility of H2O in silicate melts: quasicrystalline models. J Geol 72:601–617
Shaw HR (1974) Diffusion of H2O in granitic liquid. I. Experimental data. II. Mass transfer in magma chambers. In: Hoffmann AW, Giletti BJ, Yoder HS Jr, Yund RA (eds) Geochemical transport and kinetics. Carnegie Inst Washington Publ 634:139–170
Silver LA, Stolper E (1985) A thermodynamic model for hydrous silicate melts. J Geol 93:161–178
Silver LA, Stolper E (1989) Water in albitic glasses. J Petrol 30:667–709
Stanton TR, Holloway JR, Hervig RL, Stolper EM (1988) Isotopic investigations of water diffusion mechanism in silicate melts. I: D2O diffusion in rhyolitic melts (in preparation)
Stewart DB (1967) Four-phase curve in the system CaAl2Si2O8−SiO2−H2O between 1 and 10 kilobars. Schweiz Mineral Petrogr Mitt 47/1:35–59
Stolper EM (1982a) Water in silicate glasses: An infrared spectroscopic study. Contrib Mineral Petrol 94:178–182
Stolper EM (1982b) The speciation of water in silicate melts. Geochim Cosmochim Acta 46:2609–2620
Stolper EM (1989) The temperature dependence of the speciation of water in rhyolitic melts and glasses. Am Mineral (in press)
Stolper EM, Silver LA (1985) The speciation of water in silicate glasses: The influence of bulk composition. EOS 66:1140
Takata M, Acocella J, Tomozawa M, Watson EB (1981) Effect of water content on the electrical conductivity of Na2O·3 SiO2 glass. J Am Ceram Soc 64:719–724
Tomlinson JW (1956) A note on the solubility of water in molten sodium silicate. J Soc Glass Technol 40:25–31
Tuttle OF, Bowen NL (1958) Origin of granite in the light of experimental studies in the system NaSlSi3O8−KAlSi3O8−SiO2−H2O. Geol Soc Am Mem 74
Uys JM, King TB (1963) The effect of basicity on the solubility of water in silicate melts. Trans Met Soc AIME 227:492–500
Wu CK (1980) Nature of incorporated water in hydrated silicate glasses. J Amer Ceram Soc 63:453–457
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Silver, L.A., Ihinger, P.D. & Stolper, E. The influence of bulk composition on the speciation of water in silicate glasses. Contr. Mineral. and Petrol. 104, 142–162 (1990). https://doi.org/10.1007/BF00306439
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00306439