Abstract
Purpose
To evaluate the influence of riociguat on World Health Organization functional class (WHO FC), 6-min walk distance (6MWD), right heart remodeling, and right ventricular–pulmonary arterial (RV–PA) coupling in patients with idiopathic pulmonary arterial hypertension (IPAH) who are treatment-naïve or who have failed to achieve treatment goals with sildenafil therapy.
Methods
Twenty patients with IPAH were enrolled: 12 had not previously received PAH-targeted therapy (treatment-naïve subgroup) and 8 had been receiving sildenafil therapy but failed to achieve treatment goals; on entering this pilot study these 8 patients were switched from sildenafil to riociguat therapy (treatment-switch subgroup). Patients received riociguat individually dose-adjusted up to a maximum of 2.5 mg three times daily. After 12 weeks, patients were assessed for WHO FC, 6MWD, right heart remodeling, and RV–PA coupling.
Results
Riociguat significantly improved WHO FC in treatment-naïve patients (from 0/4/8/0 patients in WHO I/II/III/IV at baseline to 1/6/5/0 at week 12) and in treatment-switch patients (from 0/4/4/0 patients in WHO I/II/III/IV at baseline to 1/4/3/0 at week 12). Additionally, treatment-naïve and treatment-switch patients showed significant improvements at week 12 versus baseline in 6MWD (increases of + 76.8 m and + 71.6 m, respectively), RV systolic function, and RV–PA coupling.
Conclusion
These results support the proven efficacy of riociguat in patients with IPAH, including treatment-naïve patients and those switching to riociguat following failure to achieve treatment goals with sildenafil, and suggest that it may be possible to delay disease progression in this patient group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic pulmonary arterial hypertension (IPAH; primary pulmonary arterial hypertension [PAH]) develops as a result of progressive increase in pulmonary vascular resistance (PVR). This occurs as a consequence of complex pathogenic processes in the vascular wall, including inflammation, vasoconstriction, proliferation, fibrosis, and thrombosis, resulting in the obstructive reconstruction of small pulmonary arteries and arterioles. Increasing PVR leads to right ventricular (RV) dysfunction, development of RV failure, and early death.
Before the availability of drug treatments for IPAH, median survival was 2.8 years from diagnosis, and was as low as ~ 6 months for patients with a World Health Organization functional class (WHO FC) of IV at diagnosis [1]. Current research activities are focused on studying potential therapeutic targets and developing new PAH-targeted therapies. Until recently, phosphodiesterase type 5 inhibitors (PDE5is), such as sildenafil, were the only available therapies to influence the nitric oxide (NO)–soluble guanylate cyclase (sGC)–cyclic guanosine monophosphate (cGMP) molecular pathway and decrease the rate of cGMP degradation, resulting in alleviation of IPAH. However, some patients with PAH fail to achieve treatment goals despite monotherapy or combination therapy with PDE5is [2,3,4].
Production of cGMP is thought to be suboptimal in patients with PAH responding inadequately to PDE5is, which has been primarily attributed to NO insufficiency [5]. This can be corrected by direct stimulation of sGC [3]. Riociguat is the first direct sGC stimulator and has a dual mode of action: sensitizing sGC to endogenous NO by stabilizing NO–sGC binding, while also directly stimulating sGC via a separate binding site, independently of NO. Via this mechanism, riociguat restores the NO–sGC–cGMP pathway, leading to increased generation of cGMP and decreased pulmonary hypertension [6,7,8]. Theoretically, the dual mode of action of riociguat may be a key factor for a higher potency of the drug compared with PDE5is [4].
Treatment of patients with PAH aims to achieve the following therapeutic goals: WHO FC I/II; normalization of right heart size and RV function, defined as a right atrial area (RAA) < 18 cm2 and the absence of pericardial effusion; mean right atrial pressure (mRAP) < 8 mmHg; cardiac index (CI) ≥ 2.5 L/min/m2; 6-min walking distance (6MWD) > 440 m; peak oxygen consumption (VO2 peak) > 15 mL/kg/min; ventilatory equivalents for carbon dioxide (VE/VCO2 slope) < 36; and normalization of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels. The risk of 1-year mortality of patients with PAH is determined by a combination of these parameters [9, 10].
Achievement of therapeutic goals is associated with a better prognosis [10, 11]. Therefore, combination therapy is typically prescribed to patients who fail to respond adequately to initial monotherapy. However, some patients may require a more aggressive approach to achieve established therapeutic goals, e.g., upfront combination therapy at diagnosis, in accordance with current European Cardiology Society and European Respiratory Society guidelines [10, 12, 13]. A proportion of patients with PAH will fail to achieve their therapeutic goals with PDE5is, even in combination [5]. An sGC stimulator such as riociguat may be able to increase intracellular cGMP levels and achieve clinical benefits under conditions (such as NO depletion) that restrict the effectiveness of PDE5is [3, 5].
This pilot study aimed to evaluate the effect of riociguat on measures of efficacy including functional status, RV–pulmonary arterial (RV–PA) coupling, right heart remodeling, and interventricular interaction in treatment-naïve patients with IPAH and patients with IPAH failing to achieve therapeutic goals on the PDE5i sildenafil.
Materials and Methods
This was a pilot study (registration number 8/337, cardioweb.ru) in patients with a verified diagnosis of IPAH. Patients were required to be either treatment-naïve or switching treatment from sildenafil. Patients in the treatment-switch subgroup had received sildenafil (20–80 mg three times daily [TID]) before study baseline and were assessed as having an inadequate clinical response to sildenafil therapy (see Supplementary Information for further details). The decision to transition patients to riociguat was based on the national guidelines for diagnosis and treatment of pulmonary hypertension [14].
Patients were hospitalized in the Department of Pulmonary Hypertension and Heart Diseases, Scientific Research Institute of Clinical Cardiology of A.L. Myasnikov, Russian Cardiology Research and Production Complex of the Ministry of Health of Russian Federation. All patients received riociguat individually dose-adjusted using the prescribed algorithm (Fig. 1), from an initial dose of 1 mg TID up to a maximum of 2.5 mg TID, in fortnightly increments of 0.5 mg, provided their systolic blood pressure was ≥ 95 mmHg and they had no symptoms of systemic hypotension. In the treatment-switch subgroup, riociguat was initiated 24 h after the final sildenafil dose.
Assessments were performed at baseline and at week 12 of riociguat treatment and included 6MWD, Borg index, oxygen desaturation index, cardiopulmonary exercise testing with assessment of VO2 peak, VE/VCO2, and echocardiography with right heart remodeling parameters and evaluation of RV–PA coupling. Right heart catheterization (RHC) was performed at baseline only to define an inadequate response to PDE5i treatment. To limit bias, assessment of 6MWD, the cardiopulmonary exercise test, and echocardiography were conducted by independent experts who were not informed about the protocol features. For details of the methodology used for these assessments, see Supplementary Information.
Statistical analysis of the data was conducted using the Statistica v. 10.0 for Windows software package (Stat Soft lnc., USA), and allowed for both parametric and non-parametric analysis. Differences between the treatment-naïve and treatment-switch subgroups were assessed using the Mann–Whitney test (U-test), and were considered statistically significant if p < 0.05. Wilcoxon non-parametric tests were used to assess post-treatment changes versus baseline. Spearman correlation coefficients were used to determine correlations between parameters. The results of the analysis are presented here as median value and interquartile range (IQR; 25th–75th percentiles).
Results
Baseline Characteristics and Treatment
Twenty patients with IPAH (16 females, 4 males) aged 35.7–50.0 years were enrolled between May 2016 and May 2017 (Table 1). Of these, 12 were newly started on riociguat therapy (treatment-naïve subgroup) and eight were switched to riociguat following pretreatment with sildenafil (after 21.5 ± 16.0 months; treatment-switch subgroup). At baseline, 35% and 65% of patients were in WHO FC II and WHO FC III, respectively. Overall mean 6MWD was 381.5 m (IQR 322–430). In the treatment-naïve subgroup, 67% of patients were in WHO FC III and the mean 6MWD was 381 m (IQR 305–435) at baseline. In the treatment-switch subgroup, 50% of patients were in WHO FC III before both sildenafil and riociguat therapy initiation, and mean 6MWD was 371 m (IQR 329.5–428.7) at study baseline (358 m [IQR 324–398] before starting sildenafil therapy) (Table 1).
Baseline echocardiography revealed comparable right heart dilatation, RV systolic dysfunction, impairment of interventricular interaction, and RV–PA coupling between the two subgroups (Table 1). A significant direct correlation was found between RV–PA coupling and RAA (r = 0.8; p = 0.02) and VE/VCO2 slope (r = 0.7; p = 0.04). Compared with treatment-naïve patients, treatment-switch patients had significantly lower baseline medians for mPAP (p = 0.01), mRAP (p = 0.001), and PVR (p = 0.002), as measured by RHC, and a significantly higher baseline CI (p = 0.02).
Analysis of clinical, functional, and hemodynamic status found that 69% of the overall population, 75% of the treatment-naïve subgroup, and 63% of the treatment-switch subgroup had a high risk of 1-year mortality; the remaining patients were all classed as intermediate risk. RAA (78%), VO2 peak (92%), VE/VCO2 slope (80%), CI (82%), and mixed venous oxygen saturation (SvO2; 84%) values were the most common indicators of a high risk of 1-year mortality.
Over the 12-week study period, the median daily dose of riociguat was 6.8 mg [5.4–7.2] overall, and 6.0 mg [4.5–6.0] and 7.5 mg [6.3–7.5] in the treatment-naïve and treatment-switch subgroups, respectively.
Overall IPAH Group Outcomes
At week 12, patients in the overall population had achieved a significant improvement in WHO FC versus baseline (p = 0.001), with mean changes (∆) in 6MWD of + 76 m (p = 0.001), VO2 peak of + 2.3 mL/kg/min (p = 0.004), and VE/VCO2 slope of − 8.6 (p = 0.03) (Table 2; Fig. 2). Echocardiography at week 12 revealed a significant reduction in RV basal diameter (RVBD; p = 0.04), and decreases in mean systolic pulmonary artery pressure (SPAP) by 10.6 mmHg (p = 0.003) and mPAP by 6.2 mmHg (p = 0.007) versus baseline. Additionally, a significant increase in RV fractional area change (RVFAC; p = 0.04) was observed, together with reductions in RV end-diastolic volume (RVEDV; p = 0.003) and RV end-systolic volume (RVESV; p = 0.002) median values. These improvements were coupled with an improvement of the RV systolic function (as seen on three-dimensional echocardiography) (p = 0.04). Moreover, the RV–PA coupling value significantly decreased (p = 0.02) due to a decrease in PA effective arterial elastance (Ea) (p = 0.03) and an increase in RV end-systolic elastance (RV Emax; p = 0.03) (Table 2).
Subgroup Outcomes
At week 12, both the treatment-naïve and treatment-switch subgroups achieved significant improvements in WHO FC (p = 0.006 and p = 0.02, respectively) and 6MWD (mean ∆: + 76.8 m [p = 0.01] and + 71.6 m [p = 0.03], respectively). Additionally, significant improvements were seen in VO2 peak in both the treatment-naïve and treatment-switch subgroups (p = 0.02 and p = 0.049, respectively). A decrease in VE/VCO2 slope at week 12 versus baseline was seen in both the treatment-naïve and treatment-switch subgroups, but only reached statistical significance in treatment-switch patients (p = 0.1 and p = 0.04, respectively) (Table 2).
Echocardiography at week 12 demonstrated significant reductions from baseline in SPAP and mPAP in both the treatment-naïve subgroup (p = 0.03 and p = 0.007, respectively) and the treatment-switch subgroup (p = 0.02 and p = 0.007, respectively). A significant reduction in RVBD (p = 0.03) was also observed in treatment-naïve patients. Improvement in RV systolic function was seen in the treatment-naïve subgroup (RVFAC according to two-dimensional echocardiography, p = 0.002; and three-dimensional echocardiography, p = 0.03), and also in the treatment-switch subgroup according to three-dimensional echocardiography (p = 0.049). The RV–PA coupling parameter decreased in both the treatment-naïve and treatment-switch subgroups (p = 0.048 and p = 0.01, respectively) due to PA Ea decrease (p = 0.04 and p = 0.02, respectively) and RV Emax increase (p = 0.048 and p = 0.046, respectively) (Table 2).
A between-group comparison of mean changes after 12 weeks of riociguat treatment revealed significant differences between the two subgroups in ∆VE/VCO2 slope (p = 0.03), ∆RVBD (p = 0.04), and ∆RVFAC (p = 0.04), all favoring the treatment-naïve subgroup (Table 2).
Two patients (both treatment-naïve at baseline) remained at high risk of 1-year mortality after 12 weeks of riociguat therapy [10]. Dose adjustment to > 1.5 mg TID was not possible in these 2 patients because of systemic hypotension, and a second PAH-specific drug was prescribed in addition to riociguat.
Safety
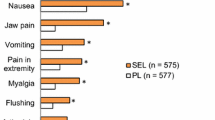
The most common adverse events were systemic hypotension (10%, n = 2), tachycardia (10%, n = 2), and exacerbation of gastroesophageal reflux disease symptoms (10%, n = 2). No adverse events required discontinuation of riociguat treatment.
Discussion
Convincing evidence of the efficacy of and tolerability of riociguat in patients with PAH has been obtained previously from the well-designed randomized, placebo-controlled phase III trial, PATENT-1 (Pulmonary Arterial Hypertension Soluble Guanylate Сyclase-Stimulator Trial 1). In PATENT-1, riociguat treatment significantly increased exercise capacity and improved a range of secondary endpoints, including hemodynamic variables, WHO FC, and time to clinical deterioration [15, 16]. The PATENT-2 long-term open-label extension trial demonstrated sustained benefits with riociguat treatment at 2 years, with an average increase of 47 m in 6MWD versus baseline, a 2-year survival rate of 93%, and clinical worsening-free survival rate of 79% [17, 18].
Transition from a PDE5i to riociguat was tested in a prospective, international, multicenter, open, non-comparative, phase IIIb trial, RESPITE (Riociguat Clinical Effects Studied in Patients with Insufficient Treatment Response to PDE5is). RESPITE evaluated the efficacy and safety of transition from sildenafil or tadalafil to riociguat in 61 patients with PAH who failed to meet treatment goals on PDE5is. The trial was designed with an 8-week riociguat dose-adjustment period followed by a 16-week period of stable treatment at a maintenance dose of riociguat up to 2.5 mg TID [3, 4]. At week 24, 52% of patients had improved from WHO FC III to WHO FC II (2% improved to WHO FC I). Other improvements included a significant increase from baseline to week 24 in mean ± SD 6MWD by + 31 ± 63 m, a decrease in PVR by − 103 ± 296 dyn s/cm5, an increase in CI by + 0.3 ± 0.5 L/min/m2, and a decrease in NT-proBNP level by − 347 ± 1235 pg/mL [4]. Currently the published evidence around transitioning patients from PDE5i to riociguat is limited to RESPITE and some case study data, while a randomized controlled trial is currently under way (Riociguat rEplacing PDE-5i Therapy evaLuated Against Continued PDE-5i thErapy [REPLACE; NCT02891850]). The findings from our pilot study show improvements in functional status and good tolerability in a small group of patients who transitioned from PDE5i to riociguat. These data add to the current pool of evidence for the use of transition as a treatment option and help to build the clinical knowledge around this area.
Cardiovascular coupling has been of special research interest in recent years. Regulation of systemic blood pressure, increase in cardiac output, and ability to respond adequately to increased heart rate all depend not only on cardiac function but also on the condition of the cardiovascular system, more precisely, on left ventricular-aortic coupling. Maintaining normal cardiovascular coupling improves the mechanic and metabolic efficiency of the cardiovascular system. Therefore, cardiovascular coupling parameters may be regarded as key factors in maintaining adequate cardiovascular performance [19, 20].
Currently, only limited information is available about the effect of PAH-specific therapy on right heart remodeling and RV–PA coupling. As RV function is a key determinant of both functional status and survival of patients with severe PAH, this is an important potential outcome for evaluating the beneficial effects of PAH-specific therapy.
Pulmonary hypertension progression leads to pressure overload and RV failure, which are followed by marked impairment in cardiovascular coupling [21]. A pilot study of interventricular interaction and RV–PA coupling has been performed in patients with IPAH of varying disease severities in the Scientific Research Institute of Clinical Cardiology of A.L. Myasnikov, Russian Cardiology Research and Production Complex. The study found that PA Ea and RV–PA coupling were significantly higher in patients with IPAH categorized as WHO FC III/IV versus those who were WHO FC I/II. A correlation analysis revealed a significant direct relationship between RV–PA coupling and RAA and a significant inverse relationship between both PA Ea and RV–PA coupling and systolic function parameters (RV ejection fraction, fractional area change, and tricuspid annular plane systolic excursion) [22].
No prior study has reported on the effects of riociguat on right heart remodeling and RV–PA coupling in either treatment-naïve patients with IPAH or patients with IPAH who have failed to achieve treatment goals with sildenafil. Our pilot study indicates that riociguat treatment may result in significant improvements in RV systolic function and decreases in RV–PA coupling for patients with IPAH, across both treatment-naïve and treatment-switch subgroups. These observations suggest that riociguat is highly effective for the treatment of patients with IPAH, and delaying disease progression in these patients may be possible.
This pilot study evaluated right heart remodeling parameters, as well as the RV–PA coupling, and their clinical and predictive value in routine practice requires further research. However, these parameters provide important information about the dynamic evaluation of the effectiveness of therapy in patients with IPAH.
This study has limitations: this was not a randomized, controlled study; the sample size was relatively small; and some clinical and laboratory data for some patient visits were not available at the time of data analysis (e.g., BNP, NT-proBNP). Additionally, RHC was not performed after 12 weeks as non-invasive criteria were considered adequate to assess response to treatment after this short period. Although the study was non-blinded, bias was limited by using independent experts who were unaware of the treatment allocations to conduct patient assessments.
In conclusion, riociguat treatment demonstrated a positive effect on functional status, RV–PA coupling, and right heart remodeling in treatment-naïve patients with IPAH and in patients with IPAH who failed to achieve treatment goals with sildenafil and who were switched to riociguat, and was well tolerated. This suggests that transitioning from sildenafil to riociguat may be an effective therapeutic strategy in patients with IPAH who fail to have an adequate treatment response to PDE5i. However, further large-scale, randomized, controlled studies are needed to confirm these initial findings.
References
D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT (1991) Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 115(5):343–349
Shapiro S, Torres F, Feldman J, Keogh A, Allard M, Blair C, Gillies H, Tislow J, Oudiz RJ (2017) Clinical and hemodynamic improvements after adding ambrisentan to background PDE5i therapy in patients with pulmonary arterial hypertension exhibiting a suboptimal therapeutic response (ATHENA-1). Respir Med 126:84–92. https://doi.org/10.1016/j.rmed.2017.03.025
Hoeper MM, Klinger JR, Benza RL, Simonneau G, Langleben D, Naeije R, Corris PA (2017) Rationale and study design of RESPITE: an open-label, phase 3b study of riociguat in patients with pulmonary arterial hypertension who demonstrate an insufficient response to treatment with phosphodiesterase-5 inhibitors. Respir Med 122(Suppl. 1):S18–S22. https://doi.org/10.1016/j.rmed.2016.11.001
Hoeper MM, Simonneau G, Corris PA, Ghofrani HA, Klinger JR, Langleben D, Naeije R, Jansa P, Rosenkranz S, Scelsi L, Grünig E, Vizza CD, Chang M, Colorado P, Meier C, Busse D, Benza RL (2017) RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. Eur Respir J 50(3):pii 1602425
Stasch JP, Pacher P, Evgenov OV (2011) Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123(20):2263–2273
Schermuly RT, Stasch JP, Pullamsetti SS, Middendorff R, Muller D, Schluter KD, Dingendorf A, Hackemack S, Kolosionek E, Kaulen C, Dumitrascu R, Weissmann N, Mittendorf J, Klepetko W, Seeger W, Ghofrani HA, Grimminger F (2008) Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J 32(4):881–891
Giaid A, Saleh D (1995) Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 333(4):214–221
Bayer AG (2017) Adempas: US prescribing information. http://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf. Accessed Nov 2017
Chazova IE, Martynyuk TV, Nakonechnikov SN (2015) Outcomes of the European Society of Cardiology Congress 2015: new version of guidelines for diagnosis and treatment of pulmonary hypertension. Evrazijskij Kardiologicheskij Zhurnal 3:3–10
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk NA, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37(1):67–119
Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J (2005) Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J 26(5):858–863. https://doi.org/10.1183/09031936.05.00075305
McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S, Palazzini M, Park MH, Tapson VF, Sitbon O (2013) Treatment goals of pulmonary hypertension. J Am Coll Cardiol 62(25 Suppl):D73–D81
Galiè N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grunig E, Oudiz RJ, Vonk-Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JH, Langley J, Rubin LJ (2015) Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 373(9):834–844
Moiseeva OM, Goncharova NS (2016) National guidelines for the diagnosis and treatment of pulmonary hypertension. Russ J Cardiol 5(133):5–64. https://doi.org/10.15829/1560-4071-2016-5-5-64
Bayer AG. Adempas. EU summary of product characteristics. http://www.emaeuropaeu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034pdf. Accessed June 2018
Ghofrani HA, Galiè N, Grimminger F, Grunig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ (2013) Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 369(4):330–340
Rubin LJ, Galiè N, Grimminger F, Grunig E, Humbert M, Jing ZC, Keogh A, Langleben D, Fritsch A, Menezes F, Davie N, Ghofrani HA (2015) Riociguat for the treatment of pulmonary arterial hypertension: a long-term extension study (PATENT-2). Eur Respir J 45(5):1303–1313
Ghofrani HA, Grimminger F, Grunig E, Huang Y, Jansa P, Jing ZC, Kilpatrick D, Langleben D, Rosenkranz S, Menezes F, Fritsch A, Nikkho S, Humbert M (2016) Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med 4:361–371
Antonini-Canterin F, Carerj S, Di Bello V, Di Salvo G, La Carrubba S, Vriz O, Pavan D, Balbarini A, Nicolosi GL, Research Group of the Italian Society of Cardiovascular Echography (SIEC) (2009) Arterial stiffness and ventricular stiffness: a couple of diseases or a coupling disease? A review from the cardiologist’s point of view. Eur J Echocardiogr 10(1):36–43. https://doi.org/10.1093/ejechocard/jen236
Antonini-Canterin F, Enache R, Popescu BA, Popescu AC, Ginghina C, Leiballi E, Piazza R, Pavan D, Rubin D, Cappelletti P, Nicolosi GL (2009) Prognostic value of ventricular-arterial coupling and B-type natriuretic peptide in patients after myocardial infarction: a five-year follow-up study. J Am Soc Echocardiogr 22(11):1239–1245. https://doi.org/10.1016/j.echo.2009.08.009
Sanz J, Garcia-Alvarez A, Fernandez-Friera L, Nair A, Mirelis JG, Sawit ST, Pinney S, Fuster V (2012) Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 98(3):238–243. https://doi.org/10.1136/heartjnl-2011-300462
Belevskaya AA (2017) Assessment of the heart structure and functional status, biventricular interdependence and caridovascular coupling in patients with pulmonary hypertension of various severity. Cand Sci (Med) Dissertation, Moscow
Acknowledgements
Writing assistance was provided by Adelphi Communications Ltd. (Bollington, UK) Writing assistance, editorial support, and article processing fees were funded by Bayer AG (Berlin, Germany). All named authors meet the ICMJE criteria for authorship of this manuscript, take responsibility for the integrity of the work, and have given approval for the final version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Taran, I.N., Belevskaya, A.A., Saidova, M.A. et al. Initial Riociguat Monotherapy and Transition from Sildenafil to Riociguat in Patients with Idiopathic Pulmonary Arterial Hypertension: Influence on Right Heart Remodeling and Right Ventricular–Pulmonary Arterial Coupling. Lung 196, 745–753 (2018). https://doi.org/10.1007/s00408-018-0160-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-018-0160-4