Abstract
Introduction
In chronic obstructive pulmonary disease (COPD), there is an activation of the l-arginine nitric oxide pathway. Pulmonary obstruction causes to elevated nitric oxide (NO) levels, which lead to higher production of the NO-inhibiting metabolites asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA).
Methods
We investigated the association of l-arginine, ADMA, and SDMA with clinical outcomes in a well-defined observational cohort of 150 patients with acute exacerbation of COPD. We measured l-arginine, ADMA, and SDMA by mass spectrometry in patients with pneumonic or non-pneumonic exacerbation of COPD included in a Swiss multicenter trial. We used Cox regression models to investigate the associations between blood marker levels and disease severity as well as all-cause mortality over a follow-up of 6.1 years.
Results
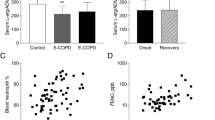
Six-year all-cause mortality was 54%. Admission levels of ADMA and SDMA (μmol L−1) were increased in 6-year non-survivors compared to survivors’ median (0.60 vs. 0.46, p = 0.004; and 1.05 vs. 0.85, p = 0.012). In a multivariate Cox regression analysis, ADMA was associated with long-term mortality resulting in an age- and comorbidity-adjusted hazard ratio (HR) of 4.55 (95% confidence interval 1.02–20.43, p = 0.048). SDMA was only associated in univariate models and no association of l-arginine with outcome was found.
Conclusion
ADMA was found to be an independent risk factor for long-term all-cause mortality in patients with acute exacerbation of COPD. Whether therapeutic modification of the l-arginine–nitric oxide pathway has the potential to improve outcome should be evaluated in future interventional trials.



Similar content being viewed by others
Abbreviations
- ADMA:
-
Asymmetric dimethylarginine
- AUC:
-
Area under the receiver operating characteristic curve
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CAP:
-
Community-acquired pneumonia
- CHF:
-
Congestive heart failure
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- CRF:
-
Chronic renal failure
- DDAH:
-
Dimethylarginine dimethylaminohydrolase
- DM:
-
Diabetes mellitus
- ED:
-
Emergency department
- eNOS:
-
Endothelial nitric oxide synthase
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- iNOS:
-
Inducible nitric oxide synthase
- LC–MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- MRM:
-
Multiple reaction monitoring
- nNOS:
-
Neuronal nitric oxide synthase
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- PCT:
-
Procalcitonin
- PRMT:
-
Protein arginine methyl transferase
- SDMA:
-
Symmetric dimethylarginine
References
Nickler M, Ottiger M, Steuer C, Huber A, Anderson JB, Muller B, Schuetz P (2015) Systematic review regarding metabolic profiling for improved pathophysiological understanding of disease and outcome prediction in respiratory infections. Respir Res 16:125. doi:10.1186/s12931-015-0283-6
Scott JA, Duongh M, Young AW, Subbarao P, Gauvreau GM, Grasemann H (2014) Asymmetric dimethylarginine in chronic obstructive pulmonary disease (ADMA in COPD). Int J Mol Sci 15(4):6062–6071. doi:10.3390/ijms15046062
Shivkar RR, Abhang SA (2014) Ratio of serum asymmetric dimethyl arginine (ADMA)/nitric oxide in coronary artery disease patients. J Clin Diag Res 8(8):CC04-06. doi:10.7860/JCDR/2014/7849.4665
Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP (2016) Alterations in whole-body arginine metabolism in chronic obstructive pulmonary disease. Am J Clin Nutr 103(6):1458–1464. doi:10.3945/ajcn.115.125187
Moncada S, Higgs A (1993) The l-arginine-nitric oxide pathway. N Engl J Med 329(27):2002–2012. doi:10.1056/NEJM199312303292706
Ruzsics I, Nagy L, Keki S, Sarosi V, Illes B, Illes Z, Horvath I, Bogar L, Molnar T (2016) l-Arginine pathway in COPD patients with acute exacerbation: a new potential biomarker. Copd 13(2):139–145. doi:10.3109/15412555.2015.1045973
Schwedhelm E, Boger RH (2011) The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol 7(5):275–285. doi:10.1038/nrneph.2011.31
Servillo L, Giovane A, Cautela D, Castaldo D, Balestrieri ML (2013) The methylarginines NMMA, ADMA, and SDMA are ubiquitous constituents of the main vegetables of human nutrition. Nitric Oxide 30:43–48. doi:10.1016/j.niox.2013.02.080
Bode-Boger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H (2006) Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol 17(4):1128–1134. doi:10.1681/ASN.2005101119
Ferrigno A, Rizzo V, Bianchi A, Di Pasqua LG, Berardo C, Richelmi P, Vairetti M (2014) Changes in ADMA/DDAH pathway after hepatic ischemia/reperfusion injury in rats: the role of bile. Biomed Res Int 2014:627434. doi:10.1155/2014/627434
Song MY, Zwemer CF, Whitesall SE, D’Alecy LG (2007) Acute and conditioned hypoxic tolerance augmented by endothelial nitric oxide synthase inhibition in mice. J Appl Physiol 102(2):610–615. doi:10.1152/japplphysiol.00894.2006
Mangoni AA (2009) The emerging role of symmetric dimethylarginine in vascular disease. Adv Clin Chem 48:73–94
Pope AJ, Karuppiah K, Cardounel AJ (2009) Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial nitric oxide production. Pharmacol Res 60(6):461–465. doi:10.1016/j.phrs.2009.07.016
Tanhauserova V, Tomandl J, Pacal L, Kleparnik M, Maluskova D, Bartakova V, Kuricova K, Rehorova J, Stepankova S, Svojanovsky J, Olsovsky J, Belobradkova J, Krusova D, Jurajda M, Muzik J, Pavlik T, Kankova K (2012) ADMA, SDMA and l-arginine/ADMA ratio but not DDAH genetic polymorphisms are reliable predictors of diabetic nephropathy progression as identified by competing risk analysis. Kidney Blood Pres Res 36(1):200–208. doi:10.1159/000343409
Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P (2003) Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23(8):1455–1459. doi:10.1161/01.ATV.0000081742.92006.59
Sibal L, Agarwal SC, Home PD, Boger RH (2010) The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev 6(2):82–90. doi:10.2174/157340310791162659
Scott JA, North ML, Rafii M, Huang H, Pencharz P, Subbarao P, Belik J, Grasemann H (2011) Asymmetric dimethylarginine is increased in asthma. Am J Respir Crit Care Med 184(7):779–785. doi:10.1164/rccm.201011-1810OC
O’Dwyer MJ, Dempsey F, Crowley V, Kelleher DP, McManus R, Ryan T (2006) Septic shock is correlated with asymmetrical dimethyl arginine levels, which may be influenced by a polymorphism in the dimethylarginine dimethylaminohydrolase II gene: a prospective observational study. Crit Care 10(5):R139. doi:10.1186/cc5053
Koch A, Weiskirchen R, Bruensing J, Duckers H, Buendgens L, Kunze J, Matthes M, Luedde T, Trautwein C, Tacke F (2013) Regulation and prognostic relevance of symmetric dimethylarginine serum concentrations in critical illness and sepsis. Mediators Inflamm 2013:413826. doi:10.1155/2013/413826
Gore MO, Luneburg N, Schwedhelm E, Ayers CR, Anderssohn M, Khera A, Atzler D, de Lemos JA, Grant PJ, McGuire DK, Boger RH (2013) Symmetrical dimethylarginine predicts mortality in the general population: observations from the Dallas heart study. Arterioscler Thromb Vasc Biol 33(11):2682–2688. doi:10.1161/ATVBAHA.113.301219
Pizzarelli F, Maas R, Dattolo P, Tripepi G, Michelassi S, D’Arrigo G, Mieth M, Bandinelli S, Ferrucci L, Zoccali C (2013) Asymmetric dimethylarginine predicts survival in the elderly. Age 35(6):2465–2475. doi:10.1007/s11357-013-9523-1
Notsu Y, Yano S, Shibata H, Nagai A, Nabika T (2015) Plasma arginine/ADMA ratio as a sensitive risk marker for atherosclerosis: Shimane CoHRE study. Atherosclerosis 239(1):61–66. doi:10.1016/j.atherosclerosis.2014.12.030
Visser M, Vermeulen MA, Richir MC, Teerlink T, Houdijk AP, Kostense PJ, Wisselink W, de Mol BA, van Leeuwen PA, Oudemans-van Straaten HM (2012) Imbalance of arginine and asymmetric dimethylarginine is associated with markers of circulatory failure, organ failure and mortality in shock patients. Br J nutr 107(10):1458–1465. doi:10.1017/S0007114511004648
Aydin M, Altintas N, Cem Mutlu L, Bilir B, Oran M, Tulubas F, Topcu B, Tayfur I, Kucukyalcin V, Kaplan G, Gurel A (2015) Asymmetric dimethylarginine contributes to airway nitric oxide deficiency in patients with COPD. Clin Respir J. doi:10.1111/crj.12337
Grolimund E, Kutz A, Marlowe RJ, Vogeli A, Alan M, Christ-Crain M, Thomann R, Falconnier C, Hoess C, Henzen C, Zimmerli W, Mueller B, Schuetz P, Pro HSG (2015) Long-term prognosis in COPD Exacerbation: role of biomarkers. Clinical variables and exacerbation type. Copd 12(3):295–305. doi:10.3109/15412555.2014.949002
Crisafulli E, Costi S, Luppi F, Cirelli G, Cilione C, Coletti O, Fabbri LM, Clini EM (2008) Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax 63(6):487–492. doi:10.1136/thx.2007.086371
Terzano C, Conti V, Di Stefano F, Petroianni A, Ceccarelli D, Graziani E, Mariotta S, Ricci A, Vitarelli A, Puglisi G, De Vito C, Villari P, Allegra L (2010) Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung 188(4):321–329. doi:10.1007/s00408-009-9222-y
Casaburi R, Celli B, Crapo J, Criner G, Croxton T, Gaw A, Jones P, Kline-Leidy N, Lomas DA, Merrill D, Polkey M, Rennard S, Sciurba F, Tal-Singer R, Stockley R, Turino G, Vestbo J, Walsh J (2013) The COPD biomarker qualification consortium (CBQC). Copd 10(3):367–377. doi:10.3109/15412555.2012.752807
Faner R, Tal-Singer R, Riley JH, Celli B, Vestbo J, MacNee W, Bakke P, Calverley PM, Coxson H, Crim C, Edwards LD, Locantore N, Lomas DA, Miller BE, Rennard SI, Wouters EF, Yates JC, Silverman EK, Agusti A, Investigators ES (2014) Lessons from ECLIPSE: a review of COPD biomarkers. Thorax 69(7):666–672. doi:10.1136/thoraxjnl-2013-204778
Schuetz P, Christ-Crain M, Wolbers M, Schild U, Thomann R, Falconnier C, Widmer I, Neidert S, Blum CA, Schonenberger R, Henzen C, Bregenzer T, Hoess C, Krause M, Bucher HC, Zimmerli W, Muller B, Hsg Pro (2007) Procalcitonin guided antibiotic therapy and hospitalization in patients with lower respiratory tract infections: a prospective, multicenter, randomized controlled trial. BMC Health Serv Res 7:102. doi:10.1186/1472-6963-7-102
Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, Neidert S, Fricker T, Blum C, Schild U, Regez K, Schoenenberger R, Henzen C, Bregenzer T, Hoess C, Krause M, Bucher HC, Zimmerli W, Mueller B, Pro HSG (2009) Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 302(10):1059–1066. doi:10.1001/jama.2009.1297
Alan M, Grolimund E, Kutz A, Christ-Crain M, Thomann R, Falconnier C, Hoess C, Henzen C, Zimmerli W, Mueller B, Schuetz P, the Pro Hsg (2014) Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: a 6 year prospective follow-up study. J Intern Med. doi:10.1111/joim.12341
Weinberger KM (2008) Metabolomics in diagnosing metabolic diseases. Therapeutische Umschau Revue therapeutique 65(9):487–491. doi:10.1024/0040-5930.65.9.487
Yet I, Menni C, Shin SY, Mangino M, Soranzo N, Adamski J, Suhre K, Spector TD, Kastenmuller G, Bell JT (2016) Genetic influences on metabolite levels: a comparison across metabolomic platforms. PLoS ONE 11(4):e0153672. doi:10.1371/journal.pone.0153672
Illig T, Gieger C, Zhai G, Romisch-Margl W, Wang-Sattler R, Prehn C, Altmaier E, Kastenmuller G, Kato BS, Mewes HW, Meitinger T, de Angelis MH, Kronenberg F, Soranzo N, Wichmann HE, Spector TD, Adamski J, Suhre K (2010) A genome-wide perspective of genetic variation in human metabolism. Nat Genet 42(2):137–141. doi:10.1038/ng.507
Guertler C, Wirz B, Christ-Crain M, Zimmerli W, Mueller B, Schuetz P (2011) Inflammatory responses predict long-term mortality risk in community-acquired pneumonia. Eur Respir J 37(6):1439–1446. doi:10.1183/09031936.00121510
Ricciardolo FL, Nijkamp FP, Folkerts G (2006) Nitric oxide synthase (NOS) as therapeutic target for asthma and chronic obstructive pulmonary disease. Curr Drug Targ 7(6):721–735
Hamad AM, Johnson SR, Knox AJ (1999) Antiproliferative effects of NO and ANP in cultured human airway smooth muscle. Am J Physiol 277(5 Pt 1):L910–L918
Patel HJ, Belvisi MG, Donnelly LE, Yacoub MH, Chung KF, Mitchell JA (1999) Constitutive expressions of type I NOS in human airway smooth muscle cells: evidence for an antiproliferative role. FASEB J 13(13):1810–1816
Allen JD (2015) A critical examination of the ergogenic/therapeutic effects of supplementation to increase nitric oxide bioavailability. Nitric Oxide 48:1–2. doi:10.1016/j.niox.2015.05.005
Tang W, Xie J, Xu S, Lv H, Lin M, Yuan S, Bai J, Hou Q, Yu S (2014) Novel nitric oxide-releasing derivatives of brusatol as anti-inflammatory agents: design, synthesis, biological evaluation, and nitric oxide release studies. J Med Chem 57(18):7600–7612. doi:10.1021/jm5007534
Lagente V, Naline E, Guenon I, Corbel M, Boichot E, Burgaud JL, Del Soldato P, Advenier C (2004) A nitric oxide-releasing salbutamol elicits potent relaxant and anti-inflammatory activities. J Pharmacol Exp Ther 310(1):367–375. doi:10.1124/jpet.103.061739
Maarsingh H, Pera T, Meurs H (2008) Arginase and pulmonary diseases. Naunyn-Schmiedeberg’s Arch Pharmacol 378(2):171–184. doi:10.1007/s00210-008-0286-7
Pera T, Zuidhof AB, Smit M, Menzen MH, Klein T, Flik G, Zaagsma J, Meurs H, Maarsingh H (2014) Arginase inhibition prevents inflammation and remodeling in a guinea pig model of chronic obstructive pulmonary disease. J Pharmacol Exp Ther 349(2):229–238. doi:10.1124/jpet.113.210138
Rathor VP, Chugh P, Ali R, Bhatnagar A, Haque SE, Bhatnagar A, Mittal G (2016) Formulation, preclinical and clinical evaluation of a new submicronic arginine respiratory fluid for treatment of chronic obstructive pulmonary disorder. Saudi Phar J 24(1):49–56. doi:10.1016/j.jsps.2015.03.010
Acknowledgements
We are thankful to the ED, medical clinic, and central laboratory staff of the University Hospital Basel and the Cantonal Hospitals of Aarau, Liestal, Lucerne, and Muensterlingen, and the ‘Buergerspital’ Solothurn for their assistance and technical support. In particular, we thank all the patients, patients’ relatives, and all local general practitioners who participated in this study. Finally, we would like to acknowledge the ProHOSP Study Group for their important support.
Funding
This study was supported in part by the Swiss National Science Foundation (SNSF Professorship, PP00P3_150531/1) and the Research Council of the Kantonsspital Aarau (1410.000.044). The initial trial was funded by the Swiss National Science Foundation (Grant SNF 3200BO-116177/1), Santé Suisse, the Gottfried and Julia Bangerter-Rhyner Foundation, and BRAHMS Biomarkers.
Author information
Authors and Affiliations
Contributions
PS, MC-C, and BM created the concept and design, wrote the protocol, and initiated the initial ProHOSP study. PS, MO, MM, and AV drafted the manuscript and performed statistical analyses. CS, LB, and AH performed laboratory measurements of the p180 Kit. All authors contributed to data acquisition, interpretation and drafting of the analyses, critical review of important content, and final approval of the manuscript. PS had full access to all data in the study and takes responsibility for the integrity of the work and the accuracy of data analysis. The ProHOSP Study Group included U. Schild, K. Regez, R. Bossart, C. Blum, M. Wolbers, S. Neidert, I. Suter, H.C. Bucher, F. Mueller, A. Chaudry, J. Haeuptle, R. Zarbosky, R. Fiumefreddo, M. Wieland, C. Nussbaumer, A. Christ, R. Bingisser, and K. Schneider (University Hospital Basel, Basel, Switzerland); T. Bregenzer, D. Conen, A. Huber, and J. Staehelin (Kantonsspital Aarau, Aarau, Switzerland); W. Zimmerli, C. Falconnier, and C. Bruehlhardt (Kantonsspital Liestal, Liestal, Switzerland); C. Henzen and V. Briner (Kantonsspital Luzern, Luzern, Switzerland); T. Fricker, C. Hoess, M. Krause, I. Lambinon, and M. Zueger (Kantonsspital Muensterlingen, Muensterlingen, Switzerland); and R. Thomann, R. Schoenenberger, and R. Luginbuehl (Buergerspital Solothurn, Solothurn, Switzerland).
Corresponding author
Ethics declarations
Conflict of interest
All authors confirm that they do not have any conflict of interest associated with this manuscript.
Ethical Approval
The ethics committee of the University of Basel approved the initial study protocol. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Vögeli, A., Ottiger, M., Meier, M.A. et al. Asymmetric Dimethylarginine Predicts Long-Term Outcome in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Lung 195, 717–727 (2017). https://doi.org/10.1007/s00408-017-0047-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-017-0047-9



