Abstract
Background
Non-small-cell lung cancer (NSCLC), the most common lung cancer, leads to the largest number of cancer-related deaths worldwide. There are many studies to identify the differentially expressed genes (DEGs) between NSCLC and normal control (NC) tissues by means of microarray technology. Because of the inconsistency of the microarray data sets, we performed an integrated analysis to identify DEGs and analyzed their biological function.
Methods and Results
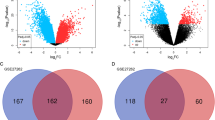
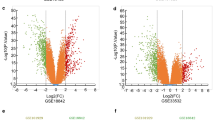
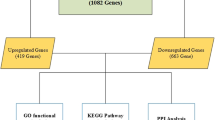
We combined 15 microarray data sets and identified 1063 DEGs between NSCLC and NC tissues; in addition, we found that the DEGs were enriched in regulation of cell proliferation process and focal adhesion signaling pathway. The protein–protein interaction network analysis for the top 20 significantly DEGs revealed that CAV1, COL1A1, and ADRB2 were the significant hub proteins. Finally, we employed qRT-PCR to validate the meta-analysis approach by determining the expression of the top 10 most significantly DEGs and found that the expression of these genes were significantly different between tumor and NC tissues, in accordance with the results of meta-analysis.
Conclusion
qRT-PCR results indicated that the meta-analysis approach in our study was acceptable. Our data suggested that some of the DEGs, including MMP12, COL11A1, THBS2, FAP, and CAV1, may participate in the pathology of NSCLC and could be applied as potential markers or therapeutic targets for NSCLC.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30
Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR et al (2013) Non-small cell lung cancer, version 2.2013. J Natl Compr Cancer Netw 11(6):645–653 quiz 53
DeRisi J, Penland L, Brown PO, Bittner ML, Meltzer PS, Ray M et al (1996) Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nat Genet 14(4):457–460
Lakhani SR, Ashworth A (2001) Microarray and histopathological analysis of tumours: the future and the past? Nat Rev Cancer 1(2):151–157
Ramasamy A, Mondry A, Holmes CC, Altman DG (2008) Key issues in conducting a meta-analysis of gene expression microarray datasets. PLoS Med 5(9):e184
Xu W, Huang H, Yu L, Cao L (2015) Meta-analysis of gene expression profiles indicates genes in spliceosome pathway are up-regulated in hepatocellular carcinoma (HCC). Med Oncol 32(4):425
Tulalamba W, Larbcharoensub N, Sirachainan E, Tantiwetrueangdet A, Janvilisri T (2015). Transcriptome meta-analysis reveals dysregulated pathways in nasopharyngeal carcinoma. Tumour biol. doi:10.1007/s13277-015-3268-7
Letellier E, Schmitz M, Baig K, Beaume N, Schwartz C, Frasquilho S et al (2014) Identification of SOCS2 and SOCS6 as biomarkers in human colorectal cancer. Br J Cancer 111(4):726–735
Yang Z, Chen Y, Fu Y, Yang Y, Zhang Y, Li D (2014) Meta-analysis of differentially expressed genes in osteosarcoma based on gene expression data. BMC Med Genet 15:80
Tabas-Madrid D, Nogales-Cadenas R, Pascual-Montano A (2012) GeneCodis3: a non-redundant and modular enrichment analysis tool for functional genomics. Nucleic Acids Res 40:W478–W483 (Web Server issue)
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13(11):2498–2504
World Health Organization (2011) Cancer: fact sheet (N297). http://www.who.int/mediacentre/factsheets/fs297/en/
Cooper GM, Hausman RE (2000) The cell. Sinauer Associates, Sunderland, pp 725–730
Dubash AD, Menold MM, Samson T, Boulter E, Garcia-Mata R, Doughman R et al (2009) Chapter 1. Focal adhesions: new angles on an old structure. Int Rev Cell Mol Biol 277:1–65
Legate KR, Fassler R (2009) Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci 122(Pt 2):187–198
Legate KR, Wickstrom SA, Fassler R (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23(4):397–418
Vehlow A, Cordes N (2013) Invasion as target for therapy of glioblastoma multiforme. Biochim Biophys Acta 1836(2):236–244
Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17(3):320–329
Bissell MJ, Kenny PA, Radisky DC (2005) Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Sym 70:343–356
Nelson CM, Bissell MJ (2006) Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 22:287–309
Gronski TJ Jr, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M et al (1997) Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem 272(18):12189–12194
Seong J, Wang N, Wang Y (2013) Mechanotransduction at focal adhesions: from physiology to cancer development. J Cell Mol Med 17(5):597–604
Hofmann HS, Hansen G, Richter G, Taege C, Simm A, Silber RE et al (2005) Matrix metalloproteinase-12 expression correlates with local recurrence and metastatic disease in non-small cell lung cancer patients. Clin Cancer Res 11(3):1086–1092
Wu YH, Chang TH, Huang YF, Huang HD, Chou CY (2014) COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene 33(26):3432–3440
Wang KK, Liu N, Radulovich N, Wigle DA, Johnston MR, Shepherd FA et al (2002) Novel candidate tumor marker genes for lung adenocarcinoma. Oncogene 21(49):7598–7604
Chong IW, Chang MY, Chang HC, Yu YP, Sheu CC, Tsai JR et al (2006) Great potential of a panel of multiple hMTH1, SPD, ITGA11 and COL11A1 markers for diagnosis of patients with non-small cell lung cancer. Oncol Rep 16(5):981–988
Metodieva SN, Nikolova DN, Cherneva RV, Dimova II, Petrov DB, Toncheva DI (2011) Expression analysis of angiogenesis-related genes in Bulgarian patients with early-stage non-small cell lung cancer. Tumori 97(1):86–94
Liu R, Li H, Liu L, Yu J, Ren X (2012) Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol Ther 13(3):123–129
Saigusa S, Toiyama Y, Tanaka K, Yokoe T, Okugawa Y, Fujikawa H et al (2011) Cancer-associated fibroblasts correlate with poor prognosis in rectal cancer after chemoradiotherapy. Int J Oncol 38(3):655–663
Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z et al (2008) Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas 37(2):154–158
Liao Y, Ni Y, He R, Liu W, Du J (2013) Clinical implications of fibroblast activation protein-alpha in non-small cell lung cancer after curative resection: a new predictor for prognosis. J Cancer Res Clin Oncol 139(9):1523–1528
Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG (1992) Caveolin, a protein component of caveolae membrane coats. Cell 68(4):673–682
Sloan EK, Stanley KL, Anderson RL (2004) Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene 23(47):7893–7897
Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ et al (2004) Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem 279(49):51630–51646
Racine C, Belanger M, Hirabayashi H, Boucher M, Chakir J, Couet J (1999) Reduction of caveolin 1 gene expression in lung carcinoma cell lines. Biochem Biophys Res Commun 255(3):580–586
Sunaga N, Miyajima K, Suzuki M, Sato M, White MA, Ramirez RD et al (2004) Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res 64(12):4277–4285
Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K et al (2001) Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol 159(5):1635–1643
Acknowledgments
This research was supported by a grant from Special project for the transformation of major scientific and technological achievements of Hebei province (No. 15277732D).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, ZQ., Li, ZH., Wen, SW. et al. Identification of Commonly Dysregulated Genes in Non-small-cell Lung Cancer by Integrated Analysis of Microarray Data and qRT-PCR Validation. Lung 193, 583–592 (2015). https://doi.org/10.1007/s00408-015-9726-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-015-9726-6