Abstract
Inhaled nitric oxide (iNO) can improve oxygenation and ventilation–perfusion (V/Q) matching by reduction of shunt (Qs/Qt) in patients with hypoxemic lung disease. Because the improvement in V/Q matching must occur by redistribution of pulmonary blood flow, and because high airway pressure (Paw) increases physiologic dead space (Vd/Vt), we hypothesized that high Paw may limit the improvement in V/Q matching during iNO treatment. iNO 0–50 ppm was administered during mechanical ventilation. Mechanical ventilator settings were at the discretion of the attending physician. Qs/Qt and Vd/Vt were derived from a tripartite lung model with correction for shunt-induced dead space. Data from 62 patients during 153 trials were analyzed for effects of Paw and iNO on Qs/Qt and Vd/Vt. Baseline Qs/Qt was slightly increased at Paw 16–23 cmH2O (p < 0.05), while Vd/Vt increased progressively with higher Paw (p < 0.002). Therapy with iNO significantly reduced Qs/Qt (p < 0.001) at all levels of mean Paw, reaching a maximum reduction at 16–23 cmH2O (p < 0.05), such that Qs/Qt during iNO treatment was similar at all levels of Paw. During iNO treatment, a reduction in Vd/Vt occurred only at Paw of 8–15 cmH2O (p < 0.05), and the positive relationship between Vd/Vt and Paw was maintained. These differential effects on Qs/Qt and Vd/Vt suggest that both high and low Paw may limit improvement in gas exchange with iNO. Analysis of gas exchange using this corrected tripartite lung model may help optimize ventilatory strategies during iNO therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inhaled nitric oxide (iNO) is used clinically to treat patients with pulmonary hypertension resulting from persistent pulmonary hypertension of the newborn (PPHN), acute respiratory distress syndrome (ARDS), and congenital heart disease [5, 6, 10, 14, 18, 23]. Physiologic responses to iNO include reduced pulmonary vascular resistance, reduced intrapulmonary shunt, increased arterial oxygenation, reduced extrapulmonary shunt, and improved cardiac output [5–7, 14, 18, 23, 31]. Because of the short half-life and high reactivity of nitric oxide with iron-containing compounds, the potent vasodilator action of nitric oxide on vascular smooth muscle is limited to those vessels adjacent to well-ventilated alveoli when nitric oxide is delivered by inhalation [23, 31]. This regional vasodilation reduces venous admixture (true shunt) and ventilation–perfusion (V/Q) heterogeneity by redistributing blood flow from poorly ventilated areas to well-ventilated areas [8, 16, 31]. This effect results in reduced total pulmonary vascular resistance with minimal systemic effects [16, 23].
Patients with pulmonary hypertension who currently qualify for iNO therapy are usually treated with mechanical ventilation [5–7, 14, 18, 23]. Mechanical ventilation has disparate effects on V/Q matching. While alveolar recruitment with mechanical ventilation increases ventilation to low-V/Q regions and transforms shunt regions to low-V/Q lung regions, the amount of high-V/Q lung region increases as a result of the combination of higher extravascular pressure and lower vascular pressure [9, 15, 27]. With optimal mean airway pressure (Paw), this redistribution of ventilation and blood flow leads to decreased venous admixture and a clinically acceptable increase in dead space. With excessive Paw, pulmonary vascular resistance may increase, potentially worsening V/Q matching, increasing extrapulmonary shunt and reducing cardiac output [4]. Therefore, the purpose of this study was to examine the effect of mean airway pressure and iNO on gas exchange parameters in a diverse group of patients with severe gas exchange impairment caused by either PPHN or ARDS. We hypothesized that positive mean airway pressure would be necessary for any beneficial effect of iNO but that excessive positive mean airway pressure would limit the redistribution of blood flow to high-V/Q areas, thereby limiting the expected beneficial effects of iNO. To examine this hypothesis, neonatal and pediatric patients with pulmonary hypertension secondary to either PPHN or ARDS were studied. Mechanical ventilation parameters were clinically optimized by the child’s physician. The redistribution of blood flow at different mean airway pressure (Paw) was probed by derivation of shunt fraction (Qs/Qt) and dead space (Vd/Vt) before and with iNO.
Patients and Methods
Patient Enrollment
Patients admitted to the Children’s Hospital of Wisconsin neonatal or pediatric intensive care units who received iNO therapy according to established protocols [18, 23] for PPHN or ARDS were included in this analysis. Institutional review boards and the United States Food and Drug Administration approved all protocols, and informed consent was obtained in all cases.
Patients with PPHN were eligible for iNO with either an oxygenation index (OI) > 40 or an alveolar–arterial difference in PO2 (AaDO2) > 600 mmHg, and pulmonary hypertension determined by extrapulmonary right-to-left shunting by either differential pulse oximetry or echocardiography. The OI was calculated as [(fiO2 × Paw)/PaO2] × 100. The AaDO2 was calculated as the alveolar PO2 (PAO2) minus the arterial PO2 (PAO2), PAO2 = [fiO2 × (743 − 47) − PaCO2/0.8)], 743 is average local barometric pressure, 47 is water vapor pressure, and 0.8 is the assumed respiratory quotient. Patients with ARDS were eligible for iNO with an OI > 13 and evidence of pulmonary hypertension by either echocardiography or direct measurement of pulmonary arterial pressure.
All patients were mechanically ventilated using either time-cycled pressure- or volume-limited devices or high-frequency oscillatory ventilation (HFOV) as clinically indicated. Ventilator management was directed by the attending physician and aimed at alveolar recruitment using low tidal volume–high airway pressure strategies to achieve adequate arterial oxygenation without overdistension. Ventilation parameters were held constant during iNO dose–response trials but were otherwise adjusted by clinical conditions.
iNO Delivery
Once patients were enrolled and consent was obtained, no further ventilator changes were made during the initial trial of iNO. The ventilator settings at the time of enrollment were at the discretion of the attending physician and determined the Paw of the patient. Patients were given iNO in doses ranging from 1 to 50 ppm according to effective dose–threshold response and delivery protocols as previously described [18, 23]. Arterial blood gas samples were obtained immediately before (baseline) and 5 min after each dose of iNO, starting at 25 ppm. The threshold for beneficial response to iNO was defined as an improvement of at least 25% in an index of arterial oxygenation [PaO2, AaDO2, the ratio of arterial to alveolar PO2 (a/AO2), OI, or Qs/Qt] compared with the off-iNO baseline. The concentration was then titrated in a logarithmic fashion (0–1–2–5–10–25–50 ppm) to find the optimum dose, defined as the minimum dose that produced a maximal or near-maximal response. Once an optimal dose was found, ventilator changes were at the discretion of the attending physician. If the maximal response was below the beneficial response threshold at all doses, iNO administration was discontinued. Dose–response trials were repeated daily to guide iNO administration at the optimum dose until iNO no longer produced a beneficial response.
Date Collection
The iNO concentration (500M, Thermo-Environmental Industries, Franklin, MA), inhaled and exhaled CO2 and O2 concentrations (Rascal-II, Ohmeda, Boulder, CO), arterial oxygenation by pulse oximetry (N-200, Nellcor, Haywood, CA), and intravascular pressures were continuously measured, digitized (DAP-102, Microstar Labs, Belleview, WA), and stored (DasyLab, DasyTec USA, Amherst, NH) on an IBM PC. The physiologic data were averaged over 20-s intervals, and 60-s running averages were continuously displayed and stored during each trial of iNO. The values of these physiologic parameters and of blood gas tensions (ABL, Radiometer America, Westlake, OH) were recorded immediately before and 5-min after each iNO dose trial. These data were used to assess the magnitude of physiologic responses for dose adjustment and to derive gas exchange were indices as follows. All data were collected prospectively using a standardized format.
Analysis of Gas Exchange
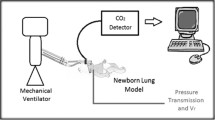
Using the data gathered above and the blood gas data, gas exchange was analyzed using a tripartite lung model with compartments for ideal alveoli, venous admixture, and true dead space as previously described [13, 17] and shown in Figure 1A. The virtual shunt (Qs/QtVirt) was computed with a widely used modification [2] of the standard formula for venous admixture. The oxygen content of arterial blood (CaO2) was derived from measured hemoglobin, arterial oxygen tension, and saturation. The O2 content of mixed pulmonary capillary blood (CcO2) was derived using the end-tidal O2 tension as the pulmonary end-capillary PO2. Given the difficulties in measuring mixed venous oxygen content in neonates with pulmonary hypertension, we used a generally accepted assumption for the arteriovenous oxygen content difference (CavO2) of 5 cc/dl to compute the virtual shunt:
(A) Schematic of tripartite lung model (ideal alveolus, shunt capillary, and dead space alveolus) for CO2 exchange. Venous blood enters the lungs via the pulmonary arteries and CO2 removal occurs only in those capillaries that perfuse ventilated alveoli. In capillaries perfusing ventilated alveoli (top capillary in the figure), the capillary PCO2 (PcCO2) approximates the alveolar PCO2 (PACO2). Dead space alveoli do not contribute CO2 to the exhaled gas (PetCO2), and shunt capillaries do not remove CO2 from the venous blood (PvCO2). Therefore, the PetCO2 is less than the PACO2 by dilution with dead space gas, and the PaCO2 is greater than the PcCO2 by admixture with venous blood. The calculation of venous admixture comes from the shunt equation [Eq. (1)], and we derive the PcCO2 using Eq. (3) from measured PaCO2, corrected PetCO2, and calculated virtual shunt. (B) Graphical representation of contributions of dead space and shunt to the end-tidal to arterial CO2 gradient as derived from Fletcher et al. [13]. Area Y is the physiologic parallel dead space, roughly proportional to the end-tidal to arterial CO2 gradient. However, part of this gradient (Y shunt) is due solely to venous admixture, to the degree that PcCO2 is different from PaCO2. The remaining component (Yalv-cor) represents the alveolar dead space corrected for the shunt effect.
Data from the time capnogram were used to approximate true alveolar dead space using methods derived from Fletcher [12, 13], as shown in Figure 1B. To yield usable data at all ventilator frequencies including HFOV, end-tidal CO2 tension (PetCO2) from the capnogram was corrected for frequency-dependent degeneration [1] by adding to the measured end-tidal CO2 tension the inspiratory baseline CO2 tension multiplied by the inspiratory time ratio [PetCO2 = PetCO2(measured) + Ti/Ttot * PiCO2 (measured)].
The physiologic Vd/Vt can be computed from PaCO2 and PetCO2 as
However, this calculation of dead space includes both alveolar dead space (high-V/Q regions) and a factitious component contributed by low-V/Q regions (effective shunt), to the extent that the PaCO2 exceeds the pulmonary capillary CO2 tension (PcCO2) by the contribution of CO2 from venous admixture (area Yshunt in Fig. 1B). The alveolar dead space as a measure of high-V/Q regions can be computed only from the ideal PcCO2. The ideal alveolar capillary PcCO2 can be expressed as a function of shunt and PaCO2 by rearrangement of the standard shunt equation [12]:
To calculate Eq. (3), the computed virtual shunt from Eq. (1) was used for Qs/Qt and a venoarterial CO2 gradient of 6 mmHg at an arteriovenous oxygen content difference of 5 cc/dl was used for PvaCO2 [11, 12].
To remove the contribution of true shunt from the calculation of alveolar dead space, the Vd/Vt was computed using the ideal alveolar capillary PcCO2 in place of PaCO2. The resulting Vd/Vt was thus corrected for the presence of intrapulmonary or extrapulmonary shunt:
These indices of shunt (Qs/Qtvirt) and alveolar dead space (Vt/Vtcor), derived from blood and end-tidal gas tensions, were used to characterize abnormalities of V/Q matching and pulmonary gas exchange [2, 12].
Statistical Analysis
Descriptive statistics are expressed as either mean ± SE (standard error) or number and percent. Differences for categorical variables were tested by the chi-squared test, and for continuous variables by one-way analysis of variance (ANOVA) or paired t-test as appropriate. Mean airway pressure was categorized into four equal-width strata (0–7, 8–15, 16–23, and 24–31 cmH2O). The effects and interaction of iNO (optimal iNO dose vs. off-iNO baseline) and airway pressure strata on indices of gas exchange were tested by a two-way ANOVA model with correction for repeated measures within trials, with post-hoc tests corrected for multiple comparisons by the Tukey wholly significant difference (wsd) method. Differences in magnitude of iNO effects at each pressure stratum were tested by ANOVA on the change in Qs/Qtvirt or Vd/Vtcor during iNO treatment, with Tukey wsd for multiple post-test comparisons. Differences were considered to be significant when p < 0.05. All calculations were performed using STATA version 6.0 (Stata Corporation, College Station, TX).
Results
Sixty-two patients were enrolled in this study. All of the patients had severe gas exchange impairment and high levels of ventilatory support [mean fiO2 = 0.87 ± 0.19 (SD) and mean Paw = 19.1 ± 6.2 (SD) cmH2O] before iNO treatment. Blood gas data, SpO2, OI, AaDO2, arterial/alveolar PO2, Qs/Qtvirt, and Vd/Vtcor at the time of study are given in Table 1. The initial effect of treatment with iNO on arterial oxygenation and gas exchange is also shown in Table 1. iNO significantly improved all of the measured and calculated clinical indices of oxygenation and gas exchange. Furthermore, as demonstrated in Table 1, iNO therapy significantly decreased both virtual shunt and alveolar dead space in these patients.
The rates of beneficial response to iNO were approximately 90% for patients with either PPHN (age = 2.5 ± 0.6 days, weight = 3.5 ± 0.1 kg, n = 42) or ARDS (age = 10 ± 2.6 years, weight 28.5 ± 3.8 kg, n = 20) (Table 2). However, patients with ARDS responded to a lower dose of iNO and required a longer period of therapy with iNO than did patients with PPHN (Table 2). In all 62 patients, the mean duration of iNO therapy was 5.4 ± 1.0 days, for a total of 228 trials of iNO therapy, yielding 820 dose–response observations and 153 optimal dose vs. off-iNO paired observations.
The baseline indices of shunt and dead space varied differentially with Paw. There was little overall difference in Qs/Qtvirt across airway pressure strata (Fig. 2; ANOVA main effect p = NS). Baseline Qs/Qtvirt was slightly greater at the mean airway pressure strata 16–23 cmH2O compared with the mean airway pressure strata <8 cmH2O, (Fig. 2; p < 0.025 by Tukey wsd). However, mean airway pressure strata had a highly significant effect on Vd/Vtcor (Fig. 3; ANOVA main effect p < 0.0001). Baseline Vd/Vtcor increased progressively from Paw <8 cmH2O to Paw >23 cmH2O (Fig. 3; all different by Tukey wsd p < 0.025).
The use of iNO therapy in these patients resulted in a significant decrease in mean Qs/Qtvirt (Table 1; ANOVA main effect p < 0.0001). While iNO reduced Qs/Qtvirt at all mean airway pressure strata, the magnitude of the reduction (negative change in Fig. 4) in Qs/Qtvirt with iNO therapy was found to be significantly greater at mean airway pressure strata 16–23 cmH2O compared with mean airway pressure strata <8 cmH2O (p < 0.05 by Tukey wsd). During iNO treatment, there were no differences in Qs/Qtvirt across airway pressure strata (p = NS, by Tukey wsd).
Treatment with iNO had a negligible effect on mean Vd/Vtcor (Table 1; ANOVA main effect p = NS). However, the interaction term was significant (p < 0.01), and stratification by mean airway pressure revealed a significant reduction (negative change in Fig. 5) in Vd/Vtcor with iNO therapy at mean airway pressure strata 8–15 cmH2O (different from all others by Tukey wsd, p < 0.05). The effect of mean airway pressure on Vd/Vtcor persisted during iNO treatment. Thus, the optimal airway pressure strata for iNO-induced reduction in dead space differed from that for reduction in shunt, and the magnitude of iNO effects on both indices of gas exchange were limited at higher airway pressure strata.
Discussion
The main findings of this study were that (1) baseline shunt was little different across airway pressures, (2) physiologic dead space increased progressively with Paw, (3) iNO therapy decreased shunt, and (4) iNO therapy had little effect on physiologic dead space. Although iNO therapy decreased shunt at all mean airway pressure strata, the effect of iNO on shunt was greatest at a mean airway pressure of 16–23 cmH2O, and the effect of iNO on dead space was restricted to a narrow range of Paw of 8–15 cmH2O. Thus, these data support our hypothesis that positive mean airway pressure is necessary to optimize the effect of iNO but that excessive positive mean airway pressure limits the redistribution of blood flow to high-V/Q areas, thereby limiting the expected beneficial effects of iNO.
While iNO treatment significantly reduced shunt at all mean airway pressure strata in this study, the effect was greatest at Paw >8 cmH2O. The premise that a mean airway pressure or a tidal volume adequate to maximize lung recruitment is necessary for a beneficial response to iNO therapy is consistent with published findings. For example, Kinsella et al. [20] found that 36% of neonates with PPHN who failed initial iNO treatment became iNO responders with the use of HFOV and increased Paw. Muller et al. [22] found that patients who responded to iNO therapy had a larger tidal volume than nonresponders. In adults with hypoxemic respiratory failure, Puybasset et al. [28] found that iNO increased PaO2 only when application of positive end-expiratory pressure had increased alveolar recruitment. Similarly, Putenson et al. [26] found that in canine lung injury, iNO at zero end-expiratory pressure had no effect on gas exchange; with CPAP and iNO, Qs/Qt decreased and perfusion of areas with normal V/Q increased. Our study suggests that the mean airway pressure range yielding the maximal iNO effect on shunt occurred in the range of 16–23 cmH2O.
The highly significant relationship between mean airway pressure and alveolar dead space suggests that higher mean airway pressures directly contribute to alveolar dead space. Treatment with iNO had less effect on alveolar dead space, with a modest reduction evident only at mean airway pressures from 8 to 15 cmH2O. Because iNO improves arterial oxygenation by redistribution of pulmonary blood flow to ventilated lung regions [26, 28], and because higher mean airway pressures increased alveolar dead space, our findings suggest that higher mean airway pressures may limit the beneficial redistribution of perfusion to regions with higher V/Q ratios, thereby also limiting the reduction in Qs/Qt.
Studies examining the effect of iNO on V/Q matching in the lungs in various diseases or models of diseases have yielded inconsistent results. On one hand, some studies have demonstrated an improvement in V/Q matching [8, 16, 24, 25]. On the other hand, some studies have shown either no effect or variable effects on V/Q matching [3, 19, 26, 30]. Alveolar recruitment and iNO response depend on adequate mean airway pressure; thus, variations in mean airway pressures and levels of alveolar recruitment may explain the differential findings in these studies of the effect of iNO on V/Q matching. Consistent with this interpretation is a recent study [21] that demonstrated that the initial response to iNO was abolished over time in a model of endotoxin administration as V/Q mismatch increased with time in pigs. Taken together these studies suggest that the level of V/Q matching may be an important determinant of response to iNO. Our findings that iNO had the greatest effect on shunt at a mean airway pressure of 16–23 cmH2O is consistent with this interpretation, because this level of mean airway pressure was associated with alveolar recruitment but was not sufficiently high to increase alveolar dead space to a degree that interfered with V/Q matching.
The mean airway pressure optimum for iNO effect will be dependent on complex interactions between underlying lung pathology, pulmonary hemodynamics, respiratory mechanics, and mechanical ventilation strategy [9, 10, 19, 20, 29]. Our study was limited in that mean airway pressure parameters for mechanical ventilation were determined by clinical indications to optimize alveolar recruitment. This strategy appears to be effectively executed in these patients based upon the relatively uniform baseline shunt across mean airway pressure strata. No ventilator adjustments were permitted during iNO trials so that the effects of iNO were tested at the clinically optimized ventilator settings only. The use of Qs/Qtvirt and Vd/Vtcor to quantify shunt and corrected alveolar dead space as indices of V/Q matching has obvious limitations compared with multicompartment models based on multiple inert gas elimination techniques. These limitations include assumptions for parameters that cannot be measured, assumption of an unchanged venoarterial PCO2 gradient in the face of probable changes in cardiac output, and assumptions underlying the partitioning of PetCO2. However, even in the face of these limitations, our findings are consistent with reported effects of IPPV and iNO on gas exchange [4, 8, 9, 15, 16, 27], and we know of no other systematic examination of the effects of iNO treatment and mean airway pressure on gas exchange using a formal lung model in this patient population. Furthermore, the parameters used in our model estimations are commonly monitored variables in the intensive care setting, and thus they may be easily applicable to patients with ARDS and PPHN in the neonatal and pediatric intensive care units, in whom pulmonary artery catheterization is rarely used.
In conclusion, we have found a significant differential interaction between mean airway pressure and iNO treatment on gas exchange indices of shunt and alveolar dead space. Alveolar recruitment with positive mean airway pressure maximized the reduction in shunt with iNO. However, higher mean airway pressures partially negated the beneficial effects of iNO therapy both by increasing baseline alveolar dead space and by limiting the improvement in V/Q matching with iNO. Thus, in this study a mean airway pressure between 8 and 23 cmH2O resulted in an optimum lung inflation range allowing for near-maximal alveolar recruitment without overdistension. Formal analysis of gas exchange using this tripartite model may help optimize ventilation strategy by guiding reduction of mean airway pressure during iNO therapy in the intensive care setting.
References
Badgwell JM, Kleinman SE, Heavner JE (1993) Respiratory frequency and artifact affect the capnographic baseline in infants. Anesth Analg 77:708–712
Benatar SR, Hewlett AM, Nunn JF (1973) The use of iso-shunt lines for control of oxygen therapy. Br J Anaesth 45:711–718
Berger Jl, Gibson RL, Redding GJ, et al. (1993) Effect of inhaled nitric oxide during group B streptococcal sepsis in piglets. Am Rev Respir Dis 147:1080–1086
Cheifetz IM, Craig DM, Quick G, et al. (1998) Increasing tidal volumes and pulmonary overdistension adversely affects pulmonary vascular mechanics and cardiac output in a pediatric swine model. Crit Care Med 26:710–716
Christou H, Van Marter LJ, Wessel DL, et al. (2000) Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Crit Care Med 28:3722–3727
Cornfield DN, Maynard RC, de Regnier RA, et al. (1999) Randomized, controlled trial of low-dose inhaled nitric oxide in the treatment of term and near-term infants with respiratory failure and pulmonary hypertension. Pediatrics 104:1089–1094
Day RW, Guarfn M, Lynch JM, Vernon DD, Dean JM (1996) Inhaled nitric oxide in children with severe lung disease: Results of acute and prolonged therapy with two concentrations. Crit Care Med 24:215–221
Dembinski R, Max M, Lopez F, et al. (2000) Effect of inhaled nitric oxide in combination with almitrine on ventilation-perfusion distribution in experimental lung injury. Intensive Care Med 26:221–228
Dembinski R, Max M, Bensberg R, et al. (2002) High frequency oscillatory ventilation in experimental lung injury: effects on gas exchange. Intensive Care Med 28:768–774
Dobyns EL, Anas NG, Fortenberry JD, et al. (2002) Interactive effects of high-frequency oscillatory ventilation and inhaled nitric oxide in acute hypoxemic respiratory failure in pediatrics. Crit Care Med 30:2425–2429
Ducey JP, Lamiell JM, Gueller GE (1992) Arterio-venous carbon dioxide tension difference during severe hemorrhage and resuscitation. Crit Care Med 20:518–522
Fletcher R (1991) The relationship between arterial to end-tidal PCO2 difference and hemoglobin saturation in patients with congenital heart disease. Anesthesiology 75:210–216
Fletcher R, Niklason L, Drefeldt B (1986) Gas exchange during controlled ventilation in children with normal and abnormal pulmonary circulation: A study using the single breath test for carbon dioxide. Anesth Analg 65:645–652
Gothberg S, Edberg KE, Tang SF, et al. (2000) Residual pulmonary hypertension in children after treatment with inhaled nitric oxide: a follow-up study regarding cardiopulmonary and neurologic symptoms. Acta Paediatr 89:1414–1419
Hillman DR (1986) Physiological aspects of intermittent positive pressure ventilation. Anaesth Inten Care 14:226–235
Hillman ND, Meliones JN, Black DR, et al. (1995) In acute lung injury, inhaled nitric oxide improves ventilation-perfusion matching, pulmonary vascular mechanics, and transpulmonary vascular efficiency. J Thorac Cardiovasc Surg 110:593–600
Hoffman GM, Torres A, Forester HV (1993) Validation of a volumeless breath-by-breath method for measurement of respiratory quotient. J Appl Physiol 75:1903–1910
Hoffman GM, Ross RA, Day SE, Rice TB, Nelin LD (1997) Inhaled nitric oxide reduces the utilization of extracorporeal membrane oxygenation in persistent pulmonary hypertension of the newborn. Crit Care Med 25:352–359
Hopkins SR, Johnson EC, Richardson RS, et al. (1997) Effects of inhaled nitric oxide on gas exchange in lungs with shunt or poorly ventilated areas. Am J Respir Crit Care Med 156:484–49120
Kinsella JP, Troug WE, Walsh WF, et al. (1997) Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr 131:55–62
Maurenbrecher H, Lamy M, Deby-Dupont G, Frascarolo P, Hedenstierna G (2001) An animal model of response and nonresponse to inhaled nitric oxide in endotoxin-induced lung injury. Chest 120:573–581
Muller W, Kachel W, Lasch P, Varnholt V, Konig SA (1996) Inhaled nitric oxide for avoidance of extracorporeal membrane oxygenation in the treatment of severe persistent pulmonary hypertension of the newborn. Intensive Care Med 22:71–76
Nelin LD, Hoffman GM (1998) The use of inhaled nitric oxide in a wide variety of clinical problems. PediatrClin North Am 45:531–538
Ogura H, Cioffi WG, Offner PJ, et al. (1994) Effect of inhaled nitric oxide on pulmonary function after sepsis in a swine model. Surgery 116:313–321
Pison U, Lopez FA, Heidelmeyer CF, Rossaint R, Falke KJ (1993) Inhaled nitric oxide reverses hypoxic pulmonary vasoconstriction without impairing gas exchange. J Appl Physiol 74:1287–1292
Putenson C, Rasanen J, Lopez FA, Down JB (1994) Continuous positive airway pressure modulates effect of inhaled nitric oxide on the ventilation-perfusion distributions in canine lung injury. Chest 106:1563–1569
Putenson C, von Spiegel T, Hering R, Stuber F, Zinserling J (1997) Effect of different ventilatory support modalities on the ventilation to perfusion distribution. Acta Anesth Scand 111:S119–S122
Puybasset L, Rouby JJ, Mourgeon E, et al. (1995) Factors influencing cardiopulmonary effects of inhaled nitric oxide in acute respiratory failure. Am J Respir Crit Care Med 152:318–328
Puybasset L, Gusman P, Muller JC, et al. (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure.CT Scan ARDS Study Group. Adult Respiratory Distress Syndrome. Intensive Care Med 26:1215–1227
Romand JA, Pinsky MR, Firestone L, Zar HA, Lancaster JR (1994) Inhaled nitric oxide partially reverses hypoxic pulmonary vasoconstriction in the dog. J Appl Physiol 76:1350–1355
Zapol WM, Rimar S, Gillis N, Marietta M, Bosken CH (1994) Nitric oxide and the lung. Am J Respir Crit Care Med 149:1375–1380
Acknowledgements
This project was funded in part by a grant from the Children’s Hospital Foundation. The authors thank the nurses and respiratory therapists in the neonatal and pediatric intensive care units at the Children’s Hospital of Wisconsin for their help and patience in carrying out these studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoffman, G.M., Nelin, L.D. Mean Airway Pressure and Response to Inhaled Nitric Oxide in Neonatal and Pediatric Patients. Lung 183, 441–453 (2005). https://doi.org/10.1007/s00408-005-2555-2
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00408-005-2555-2