Abstract
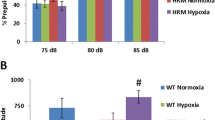
The brain-derived neurotrophic factor (BDNF) is a major proliferative agent in the nervous system. Both BDNF-deficiency and perinatal hypoxia represent genetic/environmental risk factors for schizophrenia. Moreover, a decreased BDNF response to birth hypoxia was associated with the disease. BDNF expression is influenced by neuronal activity and environmental conditions such as hypoxia. Thus, it may partake in neuroprotective and reparative mechanisms in acute or chronic neuronal insults. However, the interaction of hypoxia and BDNF is insufficiently understood and the behavioral outcome unknown. Therefore, we conducted a battery of behavioral tests in a classical model of chronic early postnatal mild hypoxia (10% O2), known to significantly impair brain development, in BDNF-deficient mice. We found selective deficits in measures associated with sensorimotor gating, namely enhanced acoustic startle response (ASR) and reduced prepulse inhibition (PPI) of ASR in BDNF-deficient mice. Unexpectedly, the alterations of sensorimotor gating were caused only by BDNF-deficiency alone, whereas hypoxia failed to evoke severe deficits and even leads to a milder phenotype in BDNF-deficient mice. As deficits in sensorimotor gating are present in schizophrenia and animal models of the disease, our results are of relevance regarding the involvement of BDNF in its pathogenesis. On the other hand, they suggest that the effect of perinatal hypoxia on long-term brain abnormalities is complex, ranging from protective to deleterious actions, and may critically depend on the degree of hypoxia. Therefore, future studies may refine existing hypoxia protocols to better understand neurodevelopmental consequences associated with schizophrenia.


Similar content being viewed by others
References
Binder DK, Scharfman HE (2004) Brain-derived neurotrophic factor. Growth Factors 22:123–131. https://doi.org/10.1016/j.bbi.2008.05.010
Tejeda GS, Diaz-Guerra M (2017) Integral characterization of defective BDNF/TrkB signalling in neurological and psychiatric disorders leads the way to new therapies. Int J Mol Sci 18:1–24. https://doi.org/10.3390/ijms18020268
Chourbaji S, Brandwein C, Gass P (2011) Altering BDNF expression by genetics and/or environment: Impact for emotional and depression-like behaviour in laboratory mice. Neurosci Biobehav Rev 35:599–611. https://doi.org/10.1016/j.neubiorev.2010.07.003
van Os J, Kapur S (2009) Schizophrenia Lancet 374:635–645. https://doi.org/10.1016/S0140-6736(09)60995-8
Brown AS (2011) The environment and susceptibility to schizophrenia. Prog Neurobiol 93:23–58. https://doi.org/10.1016/j.pneurobio.2010.09.003
Maynard TM, Sikich L, Lieberman JA, LaMantia A-S (2001) Neural Development, Cell-Cell Signaling, and the “Two-Hit” Hypothesis of Schizophrenia. Schizophr Bull 27:457–476
Quednow BB, Frommann I, Berning J et al (2008) Impaired Sensorimotor Gating of the Acoustic Startle Response in the Prodrome of Schizophrenia. Biol Psychiatry 64:766–773. https://doi.org/10.1016/j.biopsych.2008.04.019
Fendt M, Lex A, Falkai P et al (2008) Behavioural alterations in rats following neonatal hypoxia and effects of clozapine: Implications for schizophrenia. Pharmacopsychiatry 41:138–145. https://doi.org/10.1055/s-2008-1058107
Fernandes BS, Steiner J, Berk M et al (2015) Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry 20:1108–1119. https://doi.org/10.1038/mp.2014.117
Favalli G, Li J, Belmonte-de-Abreu P et al (2012) The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res 46:1–11. https://doi.org/10.1016/j.jpsychires.2011.09.022
Zornberg GL, Buka SL, Tsuang MT (2000) Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry 157:196–202. https://doi.org/10.1176/appi.ajp.157.2.196
Cannon TD, Yolken R, Buka S, Torrey EF (2008) Decreased Neurotrophic Response to Birth Hypoxia in the Etiology of Schizophrenia. Biol Psychiatry 64:797–802. https://doi.org/10.1016/j.biopsych.2008.04.012
Schmitt A, Malchow B, Hasan A, Falkai P (2014) The impact of environmental factors in severe psychiatric disorders. Front Neurosci 8:1–10. https://doi.org/10.3389/fnins.2014.00019
Bouslama M, Adla-Biassette H, Ramanantsoa N et al (2015) Protective effects of intermittent hypoxia on brain and memory in a mouse model of apnea of prematurity. Front Physiol 6:1–11. https://doi.org/10.3389/fphys.2015.00313
Xie H, Leung KL, Chen L et al (2010) Brain-derived neurotrophic factor rescues and prevents chronic intermittent hypoxia-induced impairment of hippocampal long-term synaptic plasticity. Neurobiol Dis 40:155–162. https://doi.org/10.1016/j.nbd.2010.05.020
Fagel DM, Ganat Y, Silbereis J et al (2006) Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol 199:77–91. https://doi.org/10.1016/j.expneurol.2005.04.006
Lima-Ojeda JM, Vogt MA, Richter SH et al (2014) Lack of protracted behavioral abnormalities following intermittent or continuous chronic mild hypoxia in perinatal C57BL/6 mice. Neurosci Lett 577:77–82. https://doi.org/10.1016/j.neulet.2014.06.022
Richter SH, Garner JP, Zipser B et al (2011) Effect of population heterogenization on the reproducibility of mouse behavior: a multi-laboratory study. PLoS ONE. https://doi.org/10.1371/journal.pone.0016461
Domanskyi A, Geissler C, Vinnikov IA et al (2011) Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson’s disease models. FASEB J Off Publ Fed Am Soc Exp Biol 25:2898–2910. https://doi.org/10.1096/fj.11-181958
Deacon RMJ (2006) Assessing nest building in mice. Nat Protoc 1:1117–1119
Biedermann SV, Fuss J, Steinle J et al (2016) The hippocampus and exercise: histological correlates of MR-detected volume changes. Brain Struct Funct 221:1353–1363. https://doi.org/10.1007/s00429-014-0976-5
Schneider M, Spanagel R (2008) Appetitive odor-cue conditioning attenuates the acoustic startle response in rats. Behav Brain Res 189:226–230. https://doi.org/10.1016/j.bbr.2007.12.017
Inta D, Vogt MA, Lima-Ojeda JM et al (2011) Lack of long-term behavioral alterations after early postnatal treatment with tropisetron: Implications for developmental psychobiology. Pharmacol Biochem Behav 99:35–41. https://doi.org/10.1016/j.pbb.2011.03.020
van den Buuse M (2010) Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull 36:246–270. https://doi.org/10.1093/schbul/sbp132
Pedersen CS, Sorensen DB, Parachikova AI, Plath N (2014) PCP-induced deficits in murine nest building activity: employment of an ethological rodent behavior to mimic negative-like symptoms of schizophrenia. Behav Brain Res 273:63–72. https://doi.org/10.1016/j.bbr.2014.07.023
Yeomans JS, Frankland PW (1996) The acoustic startle reflex: neurons and connections. Brain Res Rev 21:301–314
Koch M (1999) The neurobiology of startle. Prog Neurobiol 59:107–128. https://doi.org/10.1016/S0301-0082(98)00098-7
Blumenthal TD, Schicatano EJ, Chapman JG et al (1996) Prepulse effects on magnitude estimation of startle-eliciting stimuli and startle responses. Percept Psychophys 58:73–80
Swerdlow NR, Geyer MA (1998) Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull 24:285–301
Maier W, Mössner R, Quednow BB et al (2008) From genes to psychoses and back: The role of the 5HT2α-receptor and prepulse inhibition in schizophrenia. Eur Arch Psychiatry Clin Neurosci 258:40–43. https://doi.org/10.1007/s00406-008-5011-5
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156:117–154
Daenen EWPM, Wolterink G, Van Der Heyden JA et al (2003) Neonatal lesions in the amygdala or ventral hippocampus disrupt prepulse inhibition of the acoustic startle response; implications for an animal model of neurodevelopmental disorders like schizophrenia. Eur Neuropsychopharmacol 13:187–197
Naumenko VS, Bazovkina DV, Morozova MV, Popova NK (2013) Effects of brain-derived and glial cell line-derived neurotrophic factors on startle response and disrupted prepulse inhibition in mice of DBA/2J inbred strain. Neurosci Lett 550:115–118. https://doi.org/10.1016/j.neulet.2013.06.056
van den Buuse M, Lee JJW, Jaehne EJ (2017) Interaction of brain-derived neurotrophic factor Val66Met genotype and history of stress in regulation of prepulse inhibition in mice. Schizophr Res. https://doi.org/10.1016/j.schres.2017.08.019
van den Buuse M, Biel D, Radscheit K (2017) Does genetic BDNF deficiency in rats interact with neurotransmitter control of prepulse inhibition? Implications for schizophrenia. Prog Neuro 75:192–198. https://doi.org/10.1016/j.pnpbp.2017.02.009
Papaleo F, Silverman JL, Aney J et al (2011) Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. Learn Mem 18:534–544. https://doi.org/10.1101/lm.2213711
Vanevski F, Xu B (2013) Molecular and neural bases underlying roles of BDNF in the control of body weight. Front Neurosci 7:1–10. https://doi.org/10.3389/fnins.2013.00037
Chourbaji S, Hellweg R, Brandis D et al (2004) Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res 121:28–36. https://doi.org/10.1016/j.molbrainres.2003.11.002
Curzon P, Zhang M, Radek RJ, Fox GB (2009) The behavioral assessment of sensorimotor processes in the mouse: acoustic startle, sensory gating, locomotor activity. CRC Press, Boca Raton
Schmitt A, Schulenberg W, Bernstein H-G et al (2011) Reduction of gyrification index in the cerebellar vermis in schizophrenia: a post-mortem study. World J Biol Psychiatry 12(Suppl 1):99–103. https://doi.org/10.3109/15622975.2011.598379
Hirjak D, Kubera KM, Thomann PA, Wolf RC (2017) Motor dysfunction as an intermediate phenotype across schizophrenia and other psychotic disorders: progress and perspectives. Schizophr Res. https://doi.org/10.1016/j.schres.2017.10.007
Jirkof P (2014) Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods 234:139–146. https://doi.org/10.1016/j.jneumeth.2014.02.001
Bahi A (2017) Hippocampal BDNF overexpression or microR124a silencing reduces anxiety- and autism-like behaviors in rats. Behav Brain Res 326:281–290. https://doi.org/10.1016/j.bbr.2017.03.010
Gonzalez-Burgos G, Lewis DA (2012) NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 38:950–957. https://doi.org/10.1093/schbul/sbs010
Ju P, Cui D (2015) The involvement of N-methyl-d-aspartate receptor (NMDAR) subunit NR1 in the pathophysiology of schizophrenia. Acta Biochim Biophys Sin 48:209–219. https://doi.org/10.1093/abbs/gmv135 (Shanghai)
Wolf R, Dobrowolny H, Matzke K et al (2006) Prepulse inhibition is different in two inbred mouse strains (CPB-K and BALB/cJ) with different hippocampal NMDA receptor densities. Behav Brain Res 166:78–84. https://doi.org/10.1016/j.bbr.2005.07.027
Koch M, Schnitzler H-U (1997) The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav Brain Res 89:35–49. https://doi.org/10.1016/S0166-4328(97)02296-1
Gorski JA, Balogh SA, Wehner JM, Jones KR (2003) Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience 121:341–354. https://doi.org/10.1016/S0306-4522(03)00426-3
Chen S, Wu CL, Hwang WC, Yang DI (2017) More insight into BDNF against neurodegeneration: anti-apoptosis, anti-oxidation, and suppression of autophagy. Int J Mol Sci 18:1–12. https://doi.org/10.3390/ijms18030545
Samoilov M, Churilova A, Gluschenko T, Rybnikova E (2014) Neocortical pCREB and BDNF expression under different modes of hypobaric hypoxia: role in brain hypoxic tolerance in rats. Acta Histochem 116:949–957. https://doi.org/10.1016/j.acthis.2014.03.009
Kokaia Z, Zhao Q, Kokaia M et al (1995) Regulation of brain-derived neurotrophic factor gene expression after transient middle cerebral artery occlusion with and without brain damage. Exp Neurol 136:73–88. https://doi.org/10.1006/exnr.1995.1085
Ferrer I, Krupinski J, Goutan E et al (2001) Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol 101:229–238
Mattson MP (2003) Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromol Med 3:65–94. https://doi.org/10.1385/NMM:3:2:65
Ment LR, Schwartz M, Makuch RW, Stewart WB (1998) Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Dev Brain Res 111:197–203. https://doi.org/10.1016/S0165-3806(98)00139-4
Griva M, Lagoudaki R, Touloumi O et al (2017) Long-term effects of enriched environment following neonatal hypoxia-ischemia on behavior, BDNF and synaptophysin levels in rat hippocampus: effect of combined treatment with G-CSF. Brain Res 1667:55–67. https://doi.org/10.1016/j.brainres.2017.05.004
Vannucci RC, Connor JR, Mauger DT, et al (1999) Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res 55:158–163
El-Khodor BF, Boksa P (1997) Long-term reciprocal changes in dopamine levels in prefrontal cortex versus nucleus accumbens in rats born by Caesarean section compared to vaginal birth. Exp Neurol 145:118–129. https://doi.org/10.1006/exnr.1997.6437
El-Khodor B, Boksa P (2001) Caesarean section birth produces long term changes in dopamine D1 receptors and in stress-induced regulation of D3 and D4 receptors in the rat brain. Neuropsychopharmacology 25:423–439. https://doi.org/10.1016/S0893-133X(01)00228-7
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (IN168/3-1), the Ingeborg Ständer Foundation and the Research Fund of the UPK Basel to D.I.
Author information
Authors and Affiliations
Contributions
DI conceived and designed the experiments and contributed reagents/materials/analysis tools. UL contributed reagents/materials/analysis tools. JL and CB performed the experiments. JL, CB, AM and DH analyzed the data. DH, DI, AM and CB wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The manuscript does not contain clinical studies or patient data.
Rights and permissions
About this article
Cite this article
Lima-Ojeda, J.M., Mallien, A.S., Brandwein, C. et al. Altered prepulse inhibition of the acoustic startle response in BDNF-deficient mice in a model of early postnatal hypoxia: implications for schizophrenia. Eur Arch Psychiatry Clin Neurosci 269, 439–447 (2019). https://doi.org/10.1007/s00406-018-0882-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-018-0882-6