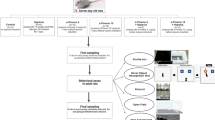
Abstract
Hypoxia is a state in which the body or a specific part of the body is deprived of adequate oxygen supply at the tissue level. Sojourners involved in different activities at high altitudes (> 2500 m) face hypobaric hypoxia (HH) due to low oxygen in the atmosphere. HH is an example of generalized hypoxia, where the homeostasis of the entire body of an organism is affected and results in neurochemical changes. It is known that lower O2 levels affect catecholamines (CA), severely impairing cognitive and locomotor behavior. However, there is less evidence on the effect of HH-mediated alteration in brain Tetrahydrobiopterin (BH4) levels and its role in neurobehavioral impairments. Hence, this study aimed to shed light on the effect of acute HH on CA and BH4 levels with its neurobehavioral impact on Wistar rat models. After HH exposure, significant alteration of the CA levels in the discrete brain regions, viz., frontal cortex, hippocampus, midbrain, and cerebellum was observed. HH exposure significantly reduced spontaneous motor activity, motor coordination, and spatial memory. The present study suggests that the HH-induced behavioral changes might be related to the alteration of the expression pattern of CA and BH4-related genes and proteins in different rat brain regions. Overall, this study provides novel insights into the role of BH4 and CA in HH-induced neurobehavioral impairments.
Graphical Abstract






Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- BH4:
-
Tetrahydrobiopterin
- CA:
-
Catecholamine
- CNS:
-
Central nervous system
- COMT:
-
Catechol-O-methyltransferase
- DA:
-
Dopamine
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DBH:
-
Dopamine-β-hydroxylase
- DDC:
-
Aromatic l-amino acid decarboxylase
- DHFR:
-
Dihydrofolate reductase
- DTT:
-
Dithiothreitol
- GCH1:
-
GTP cyclohydrolase 1
- GTP:
-
Guanosine-5'-triphosphate
- HH:
-
Hypobaric hypoxia
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- HPLC:
-
High-performance liquid chromatography
- HRP:
-
Horseradish peroxidase
- MAO:
-
Monoamine oxidase
- NE:
-
Norepinephrine
- NO:
-
Nitric oxide
- O2 :
-
Oxygen
- PD:
-
Parkinson’s disease
- PCBD1:
-
Pterin-4a-carbinolamine dehydratase 1
- PNMT:
-
Phenylethanolamine N-methyltransferase
- PTPS:
-
6-Pyruvoyltetrahydropterin synthase
- PVDF:
-
Polyvinylidene fluoride
- QDPR:
-
Quinoid dihydropteridine reductase
- SMA:
-
Spontaneous motor activity
- SPR:
-
Sepiapterin reductase
- TH:
-
Tyrosine hydroxylase
References
Williams TB, Corbett J, McMorris T, Young JS, Dicks M, Ando S, Thelwell RC, Tipton MJ, Costello JT (2019) Cognitive performance is associated with cerebral oxygenation and peripheral oxygen saturation, but not plasma catecholamines, during graded normobaric hypoxia. Exp Physiol 104(9):1384–1397. https://doi.org/10.1113/EP087647
Goldstein DS (2010) Catecholamines 101. Clin Auton Res 20(6):331–352. https://doi.org/10.1007/s10286-010-0065-7
Kobayashi K (2001) Role of catecholamine signaling in brain and nervous system functions: new insights from mouse molecular genetic study. J Investig Dermatol Symp Proc 6:115–121. https://doi.org/10.1046/j.0022-202x.2001.00011.x
Goldstein DS (2003) Catecholamines and stress. Endocr Regul 37(2):69–80 (PMID: 12932192)
Bhutta BS, Alghoula F, Berim I (2020) Anoxia (Hypoxic Hypoxia). StatPearls
Samuel J, Franklin C (2008) Hypoxemia and hypoxia. In: Myers JA et al (eds) Common surgical diseases. Springer, New York, NY, pp 391–394
Paul S, Gangwar A, Bhargava K, Khurana P, Ahmad Y (2018) Diagnosis and prophylaxis for high-altitude acclimatization: Adherence to molecular rationale to evade high-altitude illnesses. Life Sci 203:171–176. https://doi.org/10.1016/j.lfs.2018.04.040
Schneider J, Berndt N, Papageorgiou IE, Maurer J, Bulik S, Both M, Holzhütter HG, Draguhn A, Kann O (2019) Local oxygen homeostasis during various neuronal network activity states in the mouse hippocampus. J Cereb Blood Flow Metab 39(5):859–873. https://doi.org/10.1177/0271678X17740091
Alam P, Agarwal G, Kumar R, Mishra A, Saini N, Mohammad G, Pasha MQ (2020) Susceptibility to high-altitude pulmonary edema is associated with circulating miRNA levels under hypobaric hypoxia conditions. Am J Physiol Lung Cell Mol Physiol 319(2):360–368. https://doi.org/10.1152/ajplung.00168.2020
Feddersen B, Neupane P, Thanbichler F, Hadolt I, Sattelmeyer V, Pfefferkorn T, Waanders R, Noachtar S, Ausserer H (2015) Regional differences in the cerebral blood flow velocity response to hypobaric hypoxia at high altitudes. J Cereb Blood Flow Metab 35(11):1846–1851. https://doi.org/10.1038/jcbfm.2015.142
Hackett PH, Roach RC (2004) High altitude cerebral edema. High Alt Med Biol 5(2):136–146. https://doi.org/10.1089/1527029041352054
Basnyat B (1999) High-altitude cerebral edema. JAMA 281(19):1794–1794. https://doi.org/10.1089/1527029041352054
Jackson AI, Cumpstey AF, Grocott MP (2020) Acute high-altitude pathologies and their treatment. Curr Opin Endocr Metab Res 11:42–48. https://doi.org/10.1016/j.coemr.2019.12.001
Rostrup M (1998) Catecholamines, hypoxia and high altitude. Acta Physiol Scand 162(3):389–399. https://doi.org/10.1046/j.1365-201X.1998.00335.x
Flatmark T (2000) Catecholamine biosynthesis and physiological regulation in neuroendocrine cells. Acta Physiol Scand 168(1):1–18. https://doi.org/10.1046/j.1365-201x.2000.00596.x
Gnegy ME (2012) Catecholamines. In: Brady ST et al (eds) Basic neurochemistry: principles of molecular, cellular, and medical neurobiology, 8th edn. Academic press, New York
Berends A, Eisenhofer G, Fishbein L, van der Horst-Schrivers AN, Kema IP, Links TP, Lenders JW, Kerstens MN (2019) Intricacies of the molecular machinery of catecholamine biosynthesis and secretion by chromaffin cells of the normal adrenal medulla and in pheochromocytoma and paraganglioma. Cancers 11(8):1121. https://doi.org/10.3390/cancers11081121
Zumárraga M, Dávila R, Basterreche N, Arrue A, Goienetxea B, Zamalloa MI, Erkoreka L, Bustamante S, Inchausti L, González-Torres MA, Guimón J (2010) Catechol O-methyltransferase and monoamine oxidase A genotypes, and plasma catecholamine metabolites in bipolar and schizophrenic patients. Neurochem Int 56(6–7):774–779. https://doi.org/10.1016/j.neuint.2010.02.015
Kapatos G (2013) The neurobiology of tetrahydrobiopterin biosynthesis: a model for regulation of GTP cyclohydrolase I gene transcription within nigrostriatal dopamine neurons. IUBMB Life 65(4):323–333. https://doi.org/10.1002/iub.1140
Werner ER, Blau N, Thöny B (2011) Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J 438(3):397–414. https://doi.org/10.1042/BJ20110293
Thöny B, Auerbach G, Blau N (2000) Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 347(1):1–16. https://doi.org/10.1042/bj3470001
Hauton D, Holmes A, Ziff O, Kumar P (2013) The impact of acute and chronic catecholamines on respiratory responses to hypoxic stress in the rat. Pflugers Arch 465(2):209–219. https://doi.org/10.1007/s00424-012-1210-z
LaManna J, Chavez JC, Pichiule P (2004) Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol 207(18):3163–3169. https://doi.org/10.1242/jeb.00976
Bachmann LC, Matis A, Lindau NT, Felder P, Gullo M, Schwab ME (2013) Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci Transl Med 5(208):208ra146. https://doi.org/10.1126/scitranslmed.3005972
Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM (2007) Functional organization of the medial frontal cortex. Curr Opin Neurobiol 17(2):220–227. https://doi.org/10.1016/j.conb.2007.03.001
Prusky GT, Douglas RM, Nelson L, Shabanpoor A, Sutherland RJ (2004) Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. PNAS 101(14):5064–5068. https://doi.org/10.1073/pnas.0308528101
Leggio MG, Molinari M, Neri P, Graziano A, Mandolesi L, Petrosini L (2000) Representation of actions in rats: the role of cerebellum in learning spatial performances by observation. PNAS 97(5):2320–2325. https://doi.org/10.1073/pnas.040554297
Strewe C, Thieme D, Dangoisse C, Fiedel B, van den Berg F, Bauer H, Salam AP, Gössmann-Lang P, Campolongo P, Moser D, Quintens R (2018) Modulations of neuroendocrine stress responses during confinement in Antarctica and the role of hypobaric hypoxia. Front Physiol 9:1647. https://doi.org/10.3389/fphys.2018.01647
Scott AL, Pranckevicius NA, Nurse CA, Scott GR (2019) Regulation of catecholamine release from the adrenal medulla is altered in deer mice (Peromyscus maniculatus) native to high altitudes. Am J Physiol Regul Integr Comp Physiol 317(3):407–417. https://doi.org/10.1152/ajpregu.00005.2019
Sharma P, Tulsawani R (2020) Ganoderma lucidum aqueous extract prevents hypobaric hypoxia induced memory deficit by modulating neurotransmission, neuroplasticity and maintaining redox homeostasis. Sci Rep 10(1):1–16. https://doi.org/10.1038/s41598-020-65812-5
Shi Z, Vasquez-Vivar J, Luo K, Yan Y, Northington F, Mehrmohammadi M, Tan S (2018) Ascending lipopolysaccharide-induced intrauterine inflammation in near-term rabbits leading to newborn neurobehavioral deficits. Dev Neurosci 40(5–6):534–546. https://doi.org/10.1159/000499960
Molina F, Del Moral ML, Peinado MÁ, Rus A (2017) Response of the nitric oxide system to hypobaric hypoxia in the aged striatum. Gerontology 63(1):36–44. https://doi.org/10.1159/000450607
Shukla D, Saxena S, Purushothaman J, Shrivastava K, Singh M, Shukla S, Malhotra VK, Mustoori S, Bansal A (2011) Hypoxic preconditioning with cobalt ameliorates hypobaric hypoxia induced pulmonary edema in rat. Eur J Pharmacol 656(1–3):101–109. https://doi.org/10.1016/j.ejphar.2011.01.038
Whittaker VP (1965) The application of subcellular fractionation techniques to the study of brain function. Prog Biophys Mol Biol 15:39–96. https://doi.org/10.1016/0079-6107(65)90004-0
Lakshmana MK, Raju TR (1997) An isocratic assay for norepinephrine, dopamine, and 5-hydroxytryptamine using their native fluorescence by high-performance liquid chromatography with fluorescence detection in discrete brain areas of rat. Anal Biochem 246(2):166–170. https://doi.org/10.1006/abio.1996.9997
Kaneko YS, Mori K, Nakashima A, Nagatsu I, Nagatsu T, Ota A (2001) Determination of tetrahydrobiopterin in murine locus coeruleus by HPLC with fluorescence detection. Brain Res 8(1):25–31. https://doi.org/10.1016/S1385-299X(01)00081-2
Fukushima T, Nixon JC (1980) Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem 102(1):176–188. https://doi.org/10.1016/0003-2697(80)90336-X
Ameeramja J, Kanagaraj VV, Perumal E (2018) Protocatechuic acid methyl ester modulates fluoride induced pulmonary toxicity in rats. FCT 118:235–244. https://doi.org/10.1016/j.fct.2018.05.031
Fryer HJ, Davis GE, Manthorpe M, Varon S (1986) Lowry protein assay using an automatic microtiter plate spectrophotometer. Anal Biochem 153(2):262–266. https://doi.org/10.1016/0003-2697(86)90090-4
Panneerselvam L, Raghunath A, Sundarraj K, Perumal E (2019) Acute fluoride exposure alters myocardial redox and inflammatory markers in rats. Mol Biol Rep 46(6):6155–6164. https://doi.org/10.1007/s11033-019-05050-9
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11(7):36–42
Gage GJ, Kipke DR, Shain W (2012) Whole animal perfusion fixation for rodents. J Vis Exp 65:e3564. https://doi.org/10.3791/3564
Paul V, Ekambaram P, Jayakumar AR (1998) Effects of sodium fluoride on locomotor behavior and a few biochemical parameters in rats. Environ Toxicol Pharmacol 6(3):187–191. https://doi.org/10.1016/S1382-6689(98)00033-7
Dhakshinamoorthy V, Manickam V, Perumal E (2017) Neurobehavioural toxicity of iron oxide nanoparticles in mice. Neurotox Res 32(2):187–203. https://doi.org/10.1007/s12640-017-9721-1
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Rev 36(1):60–90. https://doi.org/10.1016/s0165-0173(01)00067-4
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60. https://doi.org/10.1016/0165-0270(84)90007-4
Murray AJ, Montgomery HE, Feelisch M, Grocott MP, Martin DS (2018) Metabolic adjustment to high-altitude hypoxia: from genetic signals to physiological implications. Biochem Soc Trans 46(3):599–607. https://doi.org/10.1042/BST20170502
Guo YB, He YX, Cui CY (2017) GCH1 plays a role in the high-altitude adaptation of Tibetans. Zool Res 38(3):155
Feelisch M (2018) Enhanced nitric oxide production is a universal response to hypoxic stress. Natl Sci Rev 5(4):532–533. https://doi.org/10.1161/CIRCULATIONAHA.119.040423
Lee I, Kim S, Nagar H, Choi SJ, Jeon BH, Piao S, Kim CS (2020) CR6-interacting factor 1 deficiency reduces endothelial nitric oxide synthase activity by inhibiting biosynthesis of tetrahydrobiopterin. Sci Rep 10(1):1–3. https://doi.org/10.1038/s41598-020-57673-9
Ikemoto K, Suzuki T, Ichinose H, Ohye T, Nishimura A, Nishi K, Nagatsu I, Nagatsu T (2002) Localization of sepiapterin reductase in the human brain. Brain Res 954(2):237–246. https://doi.org/10.1016/S0006-8993(02)03341-3
Milstien S, Kaufman S (1989) The biosynthesis of tetrahydrobiopterin in rat brain: purification and characterization of 6-pyruvoyl tetrahydropterin (2′-oxo) reductase. J Biol Chem 264(14):8066–8073. https://doi.org/10.1016/S0021-9258(18)83151-9
Levine RA, Kapatos G, Kaufman S, Milstien S (1990) Immunological evidence for the requirement of sepiapterin reductase for tetrahydrobiopterin biosynthesis in brain. J Neurochem 54(4):1218–1224. https://doi.org/10.1111/j.1471-4159.1990.tb01951.x
Delgado-Esteban M, Almeida A, Medina JM (2002) Tetrahydrobiopterin deficiency increases neuronal vulnerability to hypoxia. J Neurochem 82(5):1148–1159. https://doi.org/10.1046/j.1471-4159.2002.01055.x
Gozal E, Shah ZA, Pequignot JM, Pequignot J, Sachleben LR, Czyzyk-Krzeska MF, Li RC, Guo SZ, Gozal D (2005) Tyrosine hydroxylase expression and activity in the rat brain: differential regulation after long-term intermittent or sustained hypoxia. J Appl Physiol 99(2):642–649. https://doi.org/10.1152/japplphysiol.00880.2004
Mantz J, Milla C, Glowinski J, Thierry AM (1988) Differential effects of ascending neurons containing dopamine and noradrenaline in the control of spontaneous activity and of evoked responses in the rat prefrontal cortex. Neuroscience 27(2):517–526. https://doi.org/10.1016/0306-4522(88)90285-0
Knable MB, Weinberger DR (1997) Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol 11(2):123–131. https://doi.org/10.1177/026988119701100205
Lodge DJ, Grace AA (2006) The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci 103(13):5167–5172. https://doi.org/10.1073/pnas.0510715103
Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA (2006) Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res 1119(1):124–132. https://doi.org/10.1016/j.brainres.2006.08.048
Hays SA, Rennaker RL, Kilgard MP (2013) Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res 207:275–299. https://doi.org/10.1016/B978-0-444-63327-9.00010-2
Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker RL II, Kilgard MP (2013) Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis 60:80–88. https://doi.org/10.1016/j.nbd.2013.08.002
Yang BZ, Balodis IM, Lacadie CM, Xu J, Potenza MN (2016) A preliminary study of DBH (encoding dopamine beta-hydroxylase) genetic variation and neural correlates of emotional and motivational processing in individuals with and without pathological gambling. J Behav Addict 5(2):282–292. https://doi.org/10.1556/2006.5.2016.026
Fan Y, Chen P, Li Y, Zhu MY (2013) Effects of chronic social defeat on expression of dopamine β-hydroxylase in rat brains. SYNAPSE 67(6):300–312. https://doi.org/10.1002/syn.21641
Andreassi JL II, Eggleston WB, Fu G, Stewart JK (1998) Phenylethanolamine N-methyltransferase mRNA in rat hypothalamus and cerebellum. Brain Res 779(1–2):289–291. https://doi.org/10.1016/S0006-8993(97)01170-0
Jones DP (1984) Benzylamine metabolism at low O2 concentrations: relative sensitivities of monoamine oxidase, aldehyde dehydrogenase and hippurate synthesis to hypoxia. Biochem Pharmacol 33(3):413–417. https://doi.org/10.1016/0006-2952(84)90234-X
Brown RM, Kehr W, Carlsson A (1975) Functional and biochemical aspects of catecholamine metabolism in brain under hypoxia. Brain Res 85(3):491–509. https://doi.org/10.1016/0006-8993(75)90822-7
Vaccari A, Brotman S, Cimino J, Timiras PS (1978) Adaptive changes induced by high altitude in the development of brain monoamine enzymes. Neurochem Res 3(3):295–311. https://doi.org/10.1007/BF00965576
Lam CS, Li JJ, Tipoe GL, Youdim MB, Fung ML (2017) Monoamine oxidase a upregulated by chronic intermittent hypoxia activates indoleamine 2, 3-dioxygenase and neurodegeneration. PLoS ONE 12(6):e0177940. https://doi.org/10.1371/journal.pone.0177940
Romano A, Coccurello R, Giacovazzo G, Bedse G, Moles A, Gaetani S (2014) Oleoylethanolamide: a novel potential pharmacological alternative to cannabinoid antagonists for the control of appetite. Biomed Res Int 2014:203425. https://doi.org/10.1155/2014/203425
Coccurello R, D’Amato FR, Moles A (2008) Chronic administration of olanzapine affects behavioral satiety sequence and feeding behavior in female mice. Eat Weight Disord 13:e55-60
Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC (2016) Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 17(1):45–59. https://doi.org/10.1038/nrn.2015.8
Prokop P, Fančovičová J, Fedor P (2014) Parasites enhance self-grooming behaviour and information retention in humans. Behav Process 107:42–46. https://doi.org/10.1016/j.beproc.2014.07.017
Rotzinger S, Vaccarino FJ (2003) Cholecystokinin receptor subtypes: role in the modulation of anxiety-related and reward-related behaviours in animal models. J Psychiatry Neurosci 28(3):171
Berridge KC, Aldridge JW (2000) Super-stereotypy I: Enhancement of a complex movement sequence by systemic dopamine D1 agonists. SYNAPSE 37(3):194–204. https://doi.org/10.1002/1098-2396(20000901)37:3%3c194::AID-SYN3%3e3.0.CO;2-A
Berridge KC, Aldridge JW (2000) Super-stereotypy II: enhancement of a complex movement sequence by intraventricular dopamine D1 agonists. Synapse 37(3):205–215. https://doi.org/10.1002/1098-2396(20000901)37:3%3c205::AID-SYN4%3e3.0.CO;2-A
Hasbi A, O’Dowd BF, George SR (2010) Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr Opin Pharmacol 10(1):93–99. https://doi.org/10.1016/j.coph.2009.09.011
Shetty S, Pitti V, Babu CS, Kumar GS, Deepthi BC (2010) Bruxism: a literature review. J Indian Prosthodont Soc 10(3):141–148. https://doi.org/10.1007/s13191-011-0041-5
Lobbezoo F, Naeije M (2001) Bruxism is mainly regulated centrally, not peripherally. J Oral Rehabil 28(12):1085–1091. https://doi.org/10.1046/j.1365-2842.2001.00839.x
Howes OD, McCutcheon R, Owen MJ, Murray RM (2017) The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry 81(1):9–20. https://doi.org/10.1016/j.biopsych.2016.07.014
Holly EN, Miczek KA (2016) Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology 233(2):163–186. https://doi.org/10.1007/s00213-015-4151-3
Pani L, Porcella A, Gessa GL (2000) The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry 5(1):14–21. https://doi.org/10.1038/sj.mp.4000589
Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH (1996) Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci 93(3):1325–1329. https://doi.org/10.1073/pnas.93.3.1325
Clinckers R, Smolders I, Meurs A, Ebinger G, Michotte Y (2004) Anticonvulsant action of hippocampal dopamine and serotonin is independently mediated by D2 and 5-HT1A receptors. J Neurochem 89(4):834–843. https://doi.org/10.1111/j.1471-4159.2004.02355.x
Bameri B, Shaki F, Ahangar N, Ataee R, Samadi M, Mohammadi H (2018) Evidence for the involvement of the dopaminergic system in seizure and oxidative damage induced by tramadol. Int J Toxicol 37(2):164–170. https://doi.org/10.1177/1091581817753607
Globus MYT, Busto R, Dietrich WD, Martinez E, Valdés I, Ginsberg MD (1989) Direct evidence for acute and massive norepinephrine release in the hippocampus during transient ischemia. J Cereb Blood Flow Metab 9(6):892–896. https://doi.org/10.1038/jcbfm.1989.123
Collins AL, Saunders BT (2020) Heterogeneity in striatal dopamine circuits: form and function in dynamic reward seeking. J Neurosci Res 98(6):1046–1069. https://doi.org/10.1002/jnr.24587
Bromberg-Martin ES, Hikosaka O (2009) Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron 63(1):119–126. https://doi.org/10.1016/j.neuron.2009.06.009
Arias-Carrión Ó, Pöppel E (2007) Dopamine, learning, and reward-seeking behavior. Acta Neurobiol Exp 67(4):481–488
Volta M, Beccano-Kelly DA, Paschall SA, Cataldi S, MacIsaac SE, Kuhlmann N, Kadgien CA, Tatarnikov I, Fox J, Khinda J, Milnerwood AJ (2017) Initial elevations in glutamate and dopamine neurotransmission decline with age, as does exploratory behavior, in LRRK2 G2019S knock-in mice. Elife 6:e28377. https://doi.org/10.7554/eLife.28377
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Han MH (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493(7433):532–536. https://doi.org/10.1038/nature11713
Bayer HM, Glimcher PW (2005) Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 47(1):129–141. https://doi.org/10.1016/j.neuron.2005.05.020
Young CB, Reddy V, Sonne J (2020) Neuroanatomy, basal ganglia (Updated 2020 Jul 31). StatPearls Publishing, StatPearls Treasure Island, FL
Khroud NK, Reddy V, Saadabadi A (2020) Neuroanatomy, locus ceruleus. (Updated 2020 Nov 8). StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK513270/. Accessed 20 Feb 2021
Sharma Y, Xu T, Graf WM, Fobbs A, Sherwood CC, Hof PR, Allman JM, Manaye KF (2010) Comparative anatomy of the locus coeruleus in humans and nonhuman primates. J Comp Neurol 518(7):963–971. https://doi.org/10.1002/cne.22249
Bari BA, Chokshi V, Schmidt K (2020) Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease. Neural Regen Res 15(6):1006. https://doi.org/10.4103/1673-5374.270297
Mouton PR, Pakkenberg B, Gundersen HJG, Price DL (1994) Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J Chem Neuroanat 7(3):185–190. https://doi.org/10.1016/0891-0618(94)90028-0
Unsworth N, Robison MK (2017) A locus coeruleus-norepinephrine account of individual differences in working memory capacity and attention control. Psychon Bull Rev 24(4):1282–1311. https://doi.org/10.3758/s13423-016-1220-5
España RA, Schmeichel BE, Berridge CW (2016) Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res 1641:207–216. https://doi.org/10.1016/j.brainres.2016.01.002
Gabay S, Pertzov Y, Henik A (2011) Orienting of attention, pupil size, and the norepinephrine system. Atten Percept Psychophys 73(1):123–129. https://doi.org/10.3758/s13414-010-0015-4
Mückschel M, Gohil K, Ziemssen T, Beste C (2017) The norepinephrine system and its relevance for multi-component behavior. Neuroimage 146:1062–1070. https://doi.org/10.1016/j.neuroimage.2016.10.007
Khakpour-Taleghani B, Lashgari R, Motamedi F, Naghdi N (2009) Effect of reversible inactivation of locus ceruleus on spatial reference and working memory. Neuroscience 158(4):1284–1291. https://doi.org/10.1016/j.neuroscience.2008.11.001
Harlé G, Lalonde R, Fonte C, Ropars A, Frippiat JP, Strazielle C (2017) Repeated corticosterone injections in adult mice alter stress hormonal receptor expression in the cerebellum and motor coordination without affecting spatial learning. Behav Brain Res 326:121–131. https://doi.org/10.1016/j.bbr.2017.02.035
Zeng Y, She S, Lixin XU, Xuebing XU (2011) Effect of dexmedetomidine on norepinephrine release in midbrain periaqueductal gray in a rat model of incisional pain. Chin J Anesthesiol 31(3):292–295. https://doi.org/10.3760/cma.j.issn.0254-1416.2011.03.007
Gilat M, Bell PT, Martens KAE, Georgiades MJ, Hall JM, Walton CC, Lewis SJ, Shine JM (2017) Dopamine depletion impairs gait automaticity by altering cortico-striatal and cerebellar processing in Parkinson’s disease. Neuroimage 152:207–220. https://doi.org/10.1016/j.neuroimage.2017.02.073
Dirkx MF, den Ouden HE, Aarts E, Timmer MH, Bloem BR, Toni I, Helmich RC (2017) Dopamine controls Parkinson’s tremor by inhibiting the cerebellar thalamus. Brain 140(3):721–734. https://doi.org/10.1093/brain/aww331
Hurley MJ, Mash DC, Jenner P (2003) Markers for dopaminergic neurotransmission in the cerebellum in normal individuals and patients with Parkinson’s disease examined by RT-PCR. Eur J Neurosci 18(9):2668–2672. https://doi.org/10.1046/j.1460-9568.2003.02963.x
Joseph B, Nandhu MS, Paulose CS (2010) Dopamine D1 and D2 receptor functional down regulation in the cerebellum of hypoxic neonatal rats: neuroprotective role of glucose and oxygen, epinephrine resuscitation. Pharmacol Res 61(2):136–141. https://doi.org/10.1016/j.phrs.2009.08.007
Giompres P, Delis F (2005) Dopamine transporters in the cerebellum of mutant mice. Cerebellum 4(2):105–111. https://doi.org/10.1080/14734220510007851
Thach WT, Bastian AJ (2004) Role of the cerebellum in the control and adaptation of gait in health and disease. Prog Brain Res 143:353–366. https://doi.org/10.1016/s0079-6123(03)43034-3
Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB (2003) Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science 300(5624):1437–1439. https://doi.org/10.1126/science.1083661
Cooper SE, Martin JH, Ghez C (2000) Effects of inactivation of the anterior inter positus nucleus on the kinematic and dynamic control of multi joint movement. J Neurophysiol 84(4):1988–2000. https://doi.org/10.1152/jn.2000.84.4.1988
Acknowledgements
We thank Dr. K. Kadirvelu, Director, DRDO-BU CLS and Dr. T. Anand, HOD NBT Division, DFRL, Mysore, for their support. The authors would like to acknowledge DST-SERB (EMR/2014/000600) for the vibratome facility. The authors would also like to thank Dr. K. Maharajan, Dr. M. Venkataramana, Dr. S. Thenmozhi, Dr. G. Vimal, Mr. K. V. Vignesh, Mr. M. Naika, Mr. S. Teja, Ms. U. Sathisaran, Ms. M. Tomy, Ms. A. Roonie, and Mr. D. Bhattacharjee for their valuable inputs. Authors would specially like to thank Mr. Sujoy Dey (Leica Microsystems, Bengaluru) for facilitating confocal microscopy imaging at Aravind Medical Research Foundation, Madurai, Tamil Nadu, India.
Funding
This work was supported by DRDO-BU Centre for Life Sciences and funded by the Ministry of Defence, New Delhi, India for financial support (DLS/86/50011/DRDO-BU Centre/Phase-II/2014).
Author information
Authors and Affiliations
Contributions
Funding acquisition and Supervision: EP; Conceptualization: MB and EP; Methodology: MB, SM and UMD; Formal analysis and investigation: EP and SM; Writing—original draft preparation: MB; Writing—review and editing: EP and SM; Resources: EP.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
All procedures followed the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and animal ethical committee of Bharathiar University (Approval No: 722/Go/Re/S/02/CPCSEA), Coimbatore, India.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhattacharjee, M., Manoharan, S., Deshetty, U.M. et al. Acute Hypobaric Hypoxia Exposure Causes Neurobehavioral Impairments in Rats: Role of Brain Catecholamines and Tetrahydrobiopterin Alterations. Neurochem Res 48, 471–486 (2023). https://doi.org/10.1007/s11064-022-03767-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03767-x