Abstract
Purpose
Smoking, alcohol consumption, allergic rhinitis (AR), asthma, and obesity are associated with chronic rhinosinusitis (CRS), albeit the causal relationships between them remain elusive. Therefore, we conducted a bidirectional two-sample Mendelian randomization (MR) study to investigate the bidirectional causal effects between these potential risk factors and CRS.
Methods
The data for daily cigarette consumption, age of smoking initiation, weekly alcohol consumption, AR, asthma, body mass index (BMI), and CRS were drawn from large sample size genome-wide association studies. Single-nucleotide polymorphisms associated with each exposure were considered instrumental variables in this study. We investigated causal effects by using the inverse-variance weighted (IVW) method with random effects, and weighted median and MR–Egger methods were used for sensitivity analyses. Pleiotropic effects were detected and corrected by the MR pleiotropy residual sum and outlier test and MR–Egger model.
Results
We found the causal effects of daily cigarette consumption (IVW, OR = 1.15, 95% CI 1.00−1.32, p = 0.046), AR (IVW, OR = 4.77, 95% CI 1.61−14.13, p = 0.005), asthma (IVW, OR = 1.45, 95% CI 1.31 − 1.60, p < 0.001), and BMI (IVW, OR = 1.05, 95% CI 1.00−1.09, p = 0.028) on CRS. Furthermore, we found a causal effect of CRS on asthma (IVW OR = 1.08, 95% CI 1.05−1.12, p < 0.001).
Conclusions
We confirmed the causal effects of daily cigarette consumption, AR, asthma, and BMI on CRS, and the causal effect of CRS on asthma, while no causal relationship between age of smoking initiation, weekly alcohol consumption, and CRS was found. These findings are expected to provide high-quality causal evidence for clinical practice and the pathogenesis of CRS and asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is a complex chronic inflammatory disease of the sinonasal mucosa, defined by at least 3 months of nasal symptoms and objective evidence of chronic sinonasal mucosal inflammation [1, 2]. CRS can be classified into chronic rhinosinusitis with nasal polyps (CRSwNP) and chronic rhinosinusitis without nasal polyps (CRSsNP), and the main symptoms include nasal obstruction, nasal discharge, facial pain/pressure, and reduction or loss of sense of smell [3]. CRS significantly reduces patients’ quality of life and imposes a huge global economic and social burden [4, 5]. Therefore, it is necessary to investigate the potentially modifiable risk factors of CRS from an etiological standpoint and provide evidence for primary prevention and mechanism studies of CRS.
Recently, epidemiological studies have reported the association between smoking, allergic rhinitis (AR), asthma, and obesity and an increased incidence of CRS [6,7,8,9], symptoms of which can be aggravated by alcohol consumption [10]. However, observational research results are susceptible to residual confounders and reverse causality, which do not allow assertion on the causal effects of associations [11]. The causal relationships between these potential risk factors and CRS remain elusive and thus need verification. Although randomized controlled trials (RCTs) are the gold standard for causal inference, the implementation of this method is often limited by medical ethics, trial design, and research costs. It is impractical and unethical to conduct RCTs for potential risk factors and CRS. In this case, the Mendelian randomization (MR) study can overcome the above research defects and provide high-quality evidence for causal effects.
The MR study is an emerging method of investigating the causal effects of exposures (potential risk factors) on health or diseases [11]. It stimulates the design of RCTs using single-nucleotide polymorphisms (SNPs) associated with exposure as instrumental variables to investigate causal effects between exposures and disease risk [12]. It can provide reliable evidence for causal inference while coherently avoiding the effects of residual confounding and reverse causality in traditional observational studies [13]. MR studies have been gradually applied in clinical fields (such as heart diseases, diabetes, COVID-19, etc.) and have obtained reliable causal evidence. However, there is still a lack of MR studies related to CRS.
Therefore, we conducted this MR study to investigate the bidirectional causal relationships between CRS and potentially modifiable risk factors, including daily cigarette consumption, age of smoking initiation, weekly alcohol consumption, AR, asthma, and body mass index (BMI).
Materials and methods
Study design
We first identified the potential CRS-related risk factors that piqued our interest, including daily cigarette consumption, age of smoking initiation, weekly alcohol consumption, AR, asthma, and BMI. We evaluated the causal relationships between these six potential risk factors and CRS using a bidirectional two-sample MR analysis.
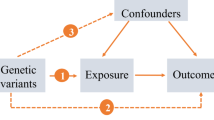
The theory behind the MR study design was that genetic variants are randomly assigned at meiosis and are independent of many other confounders, avoiding potential confounders and reverse causality and allowing reliable causal relationships to be inferred [14]. MR study design depended on the following assumptions: (1) the instrument is related with the exposure; (2) it influences the outcome only through the exposure; and (3) it is not associated with other confounders [11].
This report was based on the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) [15].
Data sources
The data of this study were drawn from results of the recent large-sample genome-wide association studies (GWAS) on people of European ancestry.
Data for CRS were obtained from the FinnGen consortium with 8524 CRS cases, obtained from hospital records (ICD-8, ICD-9, or ICD-10 codes), and 167,849 noncases. Data for daily cigarette consumption were obtained from the GWAS and Sequencing Consortium of Alcohol and Nicotine use (GSCAN) with 337,334 participants and were based on self-reports. Data for age of smoking initiation were obtained from the GSCAN with 341,427 participants and were based on self-reports. Data for weekly alcohol consumption were obtained from the GSCAN with 335,394 participants and were based on self-reports. Data for AR were obtained from the UK Biobank (UKB) with 25,486 AR cases and 87,097 noncases. The AR cases were identified from the doctor-diagnosed cases: those who answered “Yes” to the question, “Has a doctor ever told you that you have hay fever or allergic rhinitis?” were classified as AR cases. Data for asthma were obtained from the European Molecular Biology Laboratory’s European Bioinformatics Institute (EMBL–EBI) with 56,167 asthma cases and 352,555 noncases. The asthma cases were identified from the hospital records (ICD-9 or ICD-10 codes) or primary-care medical records as well as those with self-reported asthma. Data for BMI were obtained from the Within family GWAS consortium with 99,998 participants. All these original studies obtained the ethical approvals, and informed consent of all participants was collected by original researchers.
Instrumental variables (genetic variants) selection
According to the basic assumptions of the MR study, genetic variants (SNPs) associated with exposure and not associated with other confounders and outcome (except through the exposure) were selected as instrumental variables. SNPs associated with each exposure and not associated with the outcome were selected as instrumental variables at the genome-wide significance threshold of p < 5 × 10–8 when CRS was identified as the outcome. SNPs associated with the exposure and not associated with each outcome were selected as instrumental variables at the genome-wide significance threshold of p < 5 × 10–6 when CRS was identified as the exposure, since fewer SNPs reached the genome-wide significance levels of p < 5 × 10–8. We excluded all SNPs with palindromic structures and high linkage disequilibrium (r2 > 0.001). The MR pleiotropy residual sum and outlier (MR-PRESSO) test was used to detect and correct for horizontal pleiotropic outliers [16]. When instrumental variables (SNPs) independently associated with the exposure were detected in the outcome database using MR statistical methods, the exposure was assumed to have a causal effect on the outcome.
Statistics analysis
Inverse-variance weighted (IVW) method with random effects was applied as the major analysis method to estimate causal effects, which effectively overcomes the impact of heterogeneity on causal effects [17]. Weighted median (WM) and MR–Egger methods were applied for sensitivity analyses in addition to IVW [18]. When valid instrumental variables account for > 50% of the weight in the analysis, the WM method provides a reliable assessment of the causal effects between the exposure and the outcome [18]. Horizontal pleiotropy can be detected and corrected by MR–Egger method, albeit the accuracy of estimation is low [19]. We used Cochran Q statistics to assess heterogeneity, and horizontal pleiotropy was defined using the p value for the intercept of the MR–Egger model [17]. In case of no horizontal pleiotropy, the IVW method evaluation results with random effects were used as the main causal effects. In case of horizontal pleiotropy, the MR–Egger method evaluation results were used as the main causal effects. We used leave-one-out sensitivity analysis to reassess the overall causal effects by removing one SNP in succession each time.
Causal effects of exposures on outcomes were described using odds ratio (OR) and 95% confidence intervals (95% CI). To further ensure the reliability of our findings, the Bonferroni adjusted p value of significance was < 0.004 (0.05/12 = 0.004). In this study, p < 0.004 was considered as strong evidence of causal effects of exposure on outcome, whereas 0.004 < p < 0.05 was considered suggestive evidence of causal effects of exposure on outcome.
The R software version 4.1.3 (R Foundation, Vienna, Austria) was used for the selection of instrumental variables, statistics analysis, and visualization of our findings.
Results
In this study, we investigated the bidirectional causal relationships between daily cigarette consumption, age of smoking initiation, weekly alcohol consumption, AR, asthma, BMI, and CRS through the bidirectional two-sample MR analysis.
The instrumental variables we selected were at different loci and had no linkage disequilibrium (r2 < 0.001), and they were independently associated with each exposure at the genome-wide significance threshold. The F statistic of each instrumental variable we selected exceeded the threshold value of 10, indicating sufficient statistical power and effectiveness. Supplementary Tables S1 through S12 demonstrate the complete instrumental variables (SNPs) results for each trait.
MR analysis revealed suggestive causal relationships between daily cigarette consumption, AR, BMI, and CRS in forward direction (IVW, 0.004 < p < 0.05) and a significant bidirectional causal relationship between asthma and CRS (IVW, p < 0.004). The results of IVW, WM, and MR–Egger analyses are presented in Tables 1 and 2.
Causal relationships between potential risk factors and CRS in forward direction
We observed that daily cigarette consumption, AR, and BMI had suggestive causal effects on CRS, and asthma had a significant causal effect on CRS. However, no causal effect of age of smoking initiation and weekly alcohol consumption was observed on CRS. As presented in Fig. 1, the risk of CRS increased with the increase in daily cigarette consumption (IVW, OR = 1.15, 95% CI 1.00−1.32, p = 0.046). Figure 3a depicts the relationship between daily cigarette consumption and the risk of CRS. The MR–Egger model revealed no evidence of horizontal pleiotropy for the causal effect of daily cigarette consumption on CRS (intercept p = 0.201). Leave-one-out sensitivity analysis revealed that no individual SNP significantly affected the causal effect of daily cigarette consumption on CRS (Fig. S1).
The risk of CRS increased with AR (IVW, OR = 4.77, 95% CI 1.61 − 14.13, p = 0.005). Figure 3b depicts the relationship between AR and the risk of CRS. The MR-Egger model revealed no evidence of horizontal pleiotropy for the causal effect of AR on CRS (intercept p = 0.702). Leave-one-out sensitivity analysis revealed that no individual SNP significantly affected the causal effect of AR on CRS (Fig. S2).
The risk of CRS increased with asthma (IVW, OR = 1.45, 95% CI 1.31 − 1.60, p < 0.001). Figure 3c depicts the relationship between asthma and the risk of CRS. MR–Egger model revealed no evidence of horizontal pleiotropy for the causal effect of asthma on CRS (intercept p = 0.202). Leave-one-out sensitivity analysis revealed that no individual SNP significantly affected the causal effect of asthma on CRS (Fig. S3).
The risk of CRS increased with the increase in BMI (IVW, OR = 1.05, 95% CI 1.00 − 1.09, p = 0.028). Figure 3d depicts the relationship between BMI and the risk of CRS. The MR–Egger model revealed no evidence of horizontal pleiotropy for the causal effect of BMI on CRS (intercept p = 0.309). Leave-one-out sensitivity analysis revealed that no individual SNP significantly affected the causal effect of BMI on CRS (Fig. S4).
Causal relationships between potential risk factors and CRS in backward direction
We observed that CRS had a significant causal effect on asthma. No causal effect of CRS on daily cigarette consumption, age of smoking initiation, weekly alcohol consumption, AR, and BMI was observed. As presented in Fig. 2, the risk of asthma increased with CRS (IVW, OR = 1.08, 95% CI 1.05−1.12, p < 0.001). Figure 3e depicts the relationship between CRS and the risk of asthma. MR–Egger model revealed no evidence of horizontal pleiotropy for the causal effect of CRS on asthma (intercept p = 0.506). Leave-one-out sensitivity analysis revealed that no individual SNP significantly affected the causal effect of CRS on asthma (Fig. S5).
Causal relationships between potential risk factors and chronic rhinosinusitis. a Causal effect of daily cigarette consumption on chronic rhinosinusitis. b Causal effect of allergic rhinitis on chronic rhinosinusitis. c Causal effect of asthma on chronic rhinosinusitis. d Causal effect of body mass index on chronic rhinosinusitis. e Causal effect of chronic rhinosinusitis on asthma. AR allergic rhinitis, BMI body mass index, CRS chronic rhinosinusitis, SNP single-nucleotide polymorphism, MR Mendelian randomization
Discussion
This MR study demonstrated that daily cigarette consumption, AR, and BMI had suggestive causal effects on CRS and revealed a significant bidirectional causal relationship between asthma and CRS. To our knowledge, this is the first MR study to explore bidirectional causal relationships between potentially modifiable risk factors and CRS.
A systematic review by Alkholaiwi et al. presented that cigarette consumption increased the prevalence of CRS [7]. Studies have suggested a potential dose-dependent relationship between smoking and self-reported CRS [20], and smoking negatively affected the structure and function of the sinonasal mucosa [21, 22]. Our findings suggested that daily cigarette consumption had a causal effect on CRS. Smoking has been reported to increase the expression of oxidative stress and inflammatory factors [23]. In addition, studies showed that smoking was associated with increased systematic expression of IL (interleukin)-4, IL-5, and IL-13 [24, 25]. As important type 2 cytokines, IL-4, IL-5, and IL-13 play important roles in the pathogenesis of CRS, especially CRSwNP, which could be an important mechanism for the causal effect of smoking on CRS. Furthermore, sinonasal mucosa cilia injury, epithelial cell apoptosis, Staphylococcus aureus colonization and biofilm formation could also participate in the causal effect of smoking on CRS [26,27,28,29].
AR is a common comorbidity in patients with CRS. Studies reported that the positivity rate of skin prick test in patients with CRS was higher than that in control group, and patients with AR had an increased incidence of CRS [30,31,32]. Data from Geisinger Clinic primary care patients indicated a higher incidence of premorbid AR in patients with CRS [6]. Our findings suggested that AR had a causal effect on CRS, which may be through the following mechanisms: (1) the swelling of sinonasal mucosa, especially ostiomeatal complex, owing to AR leads to obstruction of the sinus orifice, causing obstructive inflammation and bacterial growth [31]. (2) AR decreases the ciliary clearance and causes ciliary loss in sinonasal mucosa [33]. (3) AR causes the increase in eosinophils, mast cells, and type 2 inflammatory factors in the nasal and sinus mucosa, participating in the occurrence of CRS, especially CRSwNP [34].
Several studies showed that asthma was closely associated with CRS. (1) A growing number of studies have reported the relation between asthma and CRS in people of all age groups [6, 8, 35]. The association was bidirectional, suggesting increased incidences of CRS in patients with asthma and asthma in patients with CRS. (2) Nasal symptoms of CRS patients with asthma were more severe and sinus radiological severity increased [36]. (3) CRS affected the severity of asthma, and treatment of CRS can reduce asthma attacks and improve symptoms [37]. However, observational studies often have many confounders that are difficult to remove, and the association of the two diseases is susceptible to reverse causality and genetic predispositions, making it hard to obtain causal inference between them. Our study provided robust evidence for the bidirectional causal relationship between asthma and CRS. With the advancement of research into airway diseases, united airways disease is becoming more widely recognized [38, 39], it is believed that there is a dynamic association between the upper and the lower airways, and respiratory inflammation may begin at the site of onset and gradually spread to other sites. It can also induce a systemic immune response by stimulating the bone marrow to produce inflammatory cells and mediators, causing a non-contact association. Based on the important roles of type 2 cytokines (e.g. IL-4, IL-5, IL-13, etc.) in the development and progression of asthma and type 2 CRS, these cytokines could be an important bridge between the two diseases in a bidirectional causal role. Furthermore, CRS may also have a causal effect on asthma through naso-bronchial reflex, respiratory pattern change, postnasal drip, etc.
A cross-sectional analysis of 229 million U.S. adults revealed the association between the increase in BMI and the increased incidence of CRS [9]. A prospective population-based study demonstrated that the incidence of new CRS was 53% higher in the obese group (BMI ≥ 30) than in the normal weight group (18.5 ≤ BMI < 25) in the Norwegian population, and higher BMI was associated with an increased risk of CRS [40]. Our study also suggested that BMI had a causal effect on CRS. Studies have revealed that obesity can cause metabolic inflammation, leading to the body being in a state of systemic low inflammation [41]. Obesity causes airway inflammation, decreased lung function, and aggravated asthma through the adipocytokines, leptin, adiponectin, other inflammatory cytokines, and mediators induced by adipose tissue [42]. According to the united airways disease theory, obesity may participate in the pathogenesis of CRS through the above-mentioned mechanisms, causing a causal effect on CRS.
Studies have reported that alcohol consumption caused upper respiratory reactions, particularly nasal obstruction and discharge, and patients with CRS often reported worsening of nasal symptoms following alcohol consumption [10, 43]. We did not observe a causal relationship between weekly alcohol consumption and CRS in either direction. Although an epidemiological survey reported that patients with CRSwNP consume more alcohol than healthy controls [44], this may be owing to uncontrollable confounders, such as increased smoking and relatively poor hygiene in patients who drink heavily. Nasal symptoms after alcohol consumption may be caused by non-specific mechanisms, such as alcohol-induced temporary vasodilation.
Although previous epidemiological studies have found associations between smoking, AR, asthma, obesity, and CRS, however, observational results have limited validity in causal inference, and are susceptible to confounders that are difficult to eliminate, making it difficult to assess causal effect sizes [12]. For example, CRS, AR, and asthma are both chronic airway diseases, the upper and lower airways are exposed to the same respiratory irritants, such as occupational exposures and environmental pollution, which may have an impact on causal effects; similar genetic predispositions between them could also influence causal inferences. Besides, smoking, drinking, and BMI often imply different occupations, health and economic conditions, and lifestyle habits, which could have an impact on the epidemiological results. Furthermore, the cross-sectional design does not allow assertion on the causal direction of associations. For the association between BMI and CRS, cross-sectional studies make it difficult to identify whether high BMI impacts CRS or BMI is impacted by CRS. CRS and asthma are also the association susceptible to bidirectional causal effects in observational studies. As an emerging research method, MR studies could avoid the limitations of the aforementioned studies and provide high-quality evidence for causality when RCTs are difficult to conduct [11]. Compared to observational studies, our MR study obtained more reliable evidence for causal effects between potential risk factors and CRS, and in addition, we have successfully assessed the causal effect sizes of these associations, which is necessary for prevention and etiological studies of CRS.
Furthermore, this study had the following strengths: we applied the two-sample MR study to investigate bidirectional causal relationships between potentially modifiable risk factors and CRS. MR studies can avoid confounders that are impossible to measure in observational studies and reverse causality, providing evidence for causal inference. We carefully checked the MR assumptions, and the data used in our study were drawn from suitable GWAS data sources with large sample sizes. The F statistic of each SNP as an instrumental variable exceeded the threshold value of 10, ensuring sufficient statistical power. We investigated causal effects using the IVW method with random effects, which effectively overcomes the impact of heterogeneity. The WM and MR–Egger methods were used as the sensitivity analyses to test the consistency of the results. In addition, the MR–PRESSO test and MR–Egger regression analysis were used to detect and correct for horizontal pleiotropy.
There were several limitations: (1) the major limitation of MR studies is that pleiotropy may affect the reliability of causal inference, although horizontal pleiotropy was not detected in our study using the MR–Egger model. (2) When evaluating the causal effect of BMI on CRS, the MR–Egger method displayed the opposite effect as the IVW and WM methods, but the MR–Egger method was not statistically significant. Since horizontal pleiotropy was not observed in the MR–Egger model, the IVW method was considered effective; however, the findings should still be explained with caution. (3) Owing to the limitation of GWAS data, we only evaluated the bidirectional causal relationships between potential risk factors and CRS and did not differentiate between CRSwNP and CRSsNP and different inflammatory endotypes. (4) This study relied on data from adults of European descent. Further research on other ethnic groups is needed. Causal inference of this study may not be applicable to children.
Conclusions
Conclusively, we demonstrated and measured the causal effects of daily cigarette consumption (OR = 1.15), AR (OR = 4.77), asthma (OR = 1.45), and BMI (OR = 1.05) on CRS, and the causal effect of CRS on asthma (OR = 1.08). However, we found no evidence supporting the causal relationship between age of smoking initiation, weekly alcohol consumption, and CRS. These findings are expected to provide high-quality causal evidence for clinical practice of rhinologists and provide a causal inference basis for the pathogenesis and prevention of CRS and asthma.
Data availiability
Data for genetic associations with potential risk factors and chronic rhinosinusitis were obtained from the FinnGen consortium, GSCAN, UK Biobank, EMBL–EBI, and the Within family GWAS consortium. Results of these orginal studies belong to public databases. Users can download relevant data for free for research (https://gwas.mrcieu.ac.uk/). Further enquiries can be directed to the corresponding author.
References
Rosenfeld RM, Piccirillo JF, Chandrasekhar SS et al (2015) Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg 152(2 Suppl):S1-s39
Sedaghat AR, Kuan EC, Scadding GK (2022) Epidemiology of chronic rhinosinusitis: Prevalence and risk factors. J Allergy Clin Immunol Pract 10(6):1395–1403
Fokkens WJ, Lund VJ, Hopkins C et al (2020) European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 58(Suppl S29):1–464
Wahid NW, Smith R, Clark A et al (2020) The socioeconomic cost of chronic rhinosinusitis study. Rhinology 58(2):112–125
Caulley L, Thavorn K, Rudmik L et al (2015) Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: results of the us medical expenditure panel survey. J Allergy Clin Immunol 136(6):1517–1522
Tan BK, Chandra RK, Pollak J et al (2013) Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol 131(5):1350–1360
Alkholaiwi FM, Almutairi RR, Alrajhi DM et al (2022) Occupational and environmental exposures, the association with chronic sinusitis. Saudi Med J 43(2):125–131
Jarvis D, Newson R, Lotvall J et al (2012) Asthma in adults and its association with chronic rhinosinusitis: the ga2len survey in europe. Allergy 67(1):91–98
Bhattacharyya N (2013) Associations between obesity and inflammatory sinonasal disorders. Laryngoscope 123(8):1840–1844
Glicksman JT, Parasher AK, Doghramji L et al (2018) Alcohol-induced respiratory symptoms improve after aspirin desensitization in patients with aspirin-exacerbated respiratory disease. Int Forum Allergy Rhinol 8(10):1093–1097
Davies NM, Holmes MV, Davey Smith G (2018) Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362:k601
Richmond RC, Davey Smith G (2022) Mendelian randomization: Concepts and scope. Cold Spring Harb Perspect Med 12(1):a040501
Lawlor DA, Harbord RM, Sterne JA et al (2008) Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat Med 27(8):1133–1163
Davey Smith G, Ebrahim S (2005) What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ 330(7499):1076–1079
Skrivankova VW, Richmond RC, Woolf BAR et al (2021) Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The strobe-mr statement. JAMA 326(16):1614–1621
Verbanck M, Chen CY, Neale B et al (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5):693–698
Burgess S, Bowden J, Fall T et al (2017) Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology 28(1):30–42
Bowden J, Davey Smith G, Haycock PC et al (2016) Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40(4):304–314
Burgess S, Thompson SG (2017) Interpreting findings from mendelian randomization using the mr-egger method. Eur J Epidemiol 32(5):377–389
Reh DD, Higgins TS, Smith TL (2012) Impact of tobacco smoke on chronic rhinosinusitis: a review of the literature. Int Forum Allergy Rhinol 2(5):362–369
Elwany S, Shewel Y, Bazak R et al (2020) Quitting smoking reverses nasal mucosal changes. Eur Arch Otorhinolaryngol 277(6):1691–1698
Tamashiro E, Cohen NA, Palmer JN et al (2009) Effects of cigarette smoking on the respiratory epithelium and its role in the pathogenesis of chronic rhinosinusitis. Braz J Otorhinolaryngol 75(6):903–907
Batatinha HAP, Rosa Neto JC, Krüger K (2019) Inflammatory features of obesity and smoke exposure and the immunologic effects of exercise. Exerc Immunol Rev 25:96–111
Cozen W, Diaz-Sanchez D, James Gauderman W et al (2004) Th1 and th2 cytokines and ige levels in identical twins with varying levels of cigarette consumption. J Clin Immunol 24(6):617–622
Byron KA, Varigos GA, Wootton AM (1994) Il-4 production is increased in cigarette smokers. Clin Exp Immunol 95(2):333–336
Brook I, Hausfeld JN (2011) Microbiology of acute and chronic maxillary sinusitis in smokers and nonsmokers. Ann Otol Rhinol Laryngol 120(11):707–712
Lee HS, Kim J (2013) Cigarette smoke inhibits nasal airway epithelial cell growth and survival. Int Forum Allergy Rhinol 3(3):188–192
Berania I, Endam LM, Filali-Mouhim A et al (2014) Active smoking status in chronic rhinosinusitis is associated with higher serum markers of inflammation and lower serum eosinophilia. Int Forum Allergy Rhinol 4(5):347–352
Shi L, Wu Y, Yang C et al (2019) Effect of nicotine on Staphylococcus aureus biofilm formation and virulence factors. Sci Rep 9(1):20243
Gutman M, Torres A, Keen KJ et al (2004) Prevalence of allergy in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg 130(5):545–552
Georgalas C, Vlastos I, Picavet V et al (2014) Is chronic rhinosinusitis related to allergic rhinitis in adults and children? Applying epidemiological guidelines for causation. Allergy 69(7):828–833
Hoffmans R, Wagemakers A, van Drunen C et al (2018) Acute and chronic rhinosinusitis and allergic rhinitis in relation to comorbidity, ethnicity and environment. PLoS ONE 13(2):e0192330
Davidson AE, Miller SD, Settipane RJ et al (1992) Delayed nasal mucociliary clearance in patients with nonallergic rhinitis and nasal eosinophilia. Allergy Proc 13(2):81–84
Cheng KJ, Zhou ML, Xu YY et al (2017) The role of local allergy in the nasal inflammation. Eur Arch Otorhinolaryngol 274(9):3275–3281
Hirsch AG, Yan XS, Sundaresan AS et al (2015) Five-year risk of incident disease following a diagnosis of chronic rhinosinusitis. Allergy 70(12):1613–1621
Shaw DE, Sousa AR, Fowler SJ et al (2015) Clinical and inflammatory characteristics of the European u-biopred adult severe asthma cohort. Eur Respir J 46(5):1308–1321
Phillips KM, Hoehle LP, Caradonna DS et al (2016) Association of severity of chronic rhinosinusitis with degree of comorbid asthma control. Ann Allergy Asthma Immunol 117(6):651–654
Samitas K, Carter A, Kariyawasam HH et al (2018) Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: the one airway concept revisited. Allergy 73(5):993–1002
Mullol J, Maldonado M, Castillo JA et al (2022) Management of united airway disease focused on patients with asthma and chronic rhinosinusitis with nasal polyps: a systematic review. J Allergy Clin Immunol Pract 10(9):2438–2447.e9
Clarhed UKE, Schiöler L, Torén K et al (2022) Bmi as a risk factor for the development of chronic rhinosinusitis: A prospective population-based study. Eur Arch Otorhinolaryngol 279(10):4953–4959
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Miethe S, Karsonova A, Karaulov A et al (2020) Obesity and asthma. J Allergy Clin Immunol 146(4):685–693
De Schryver E, Derycke L, Campo P et al (2017) Alcohol hyper-responsiveness in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy 47(2):245–253
Ahn JC, Kim JW, Lee CH et al (2016) Prevalence and risk factors of chronic rhinosinusitus, allergic rhinitis, and nasal septal deviation: results of the korean national health and nutrition survey 2008–2012. JAMA Otolaryngol Head Neck Surg 142(2):162–167
Acknowledgements
Data for genetic associations with potential risk factors and chronic rhinosinusitis were obtained from the FinnGen consortium, GSCAN, UK Biobank, EMBL–EBI, and the Within family GWAS consortium. We would like to acknowledge the participants and investigators of the FinnGen consortium, GSCAN, UK Biobank, EMBL–EBI, and the Within family GWAS consortium for providing related SNPs and GWAS data. We also thank MJEditor (www.mjeditor.com) for the English language editing and review services.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81770978), Key Technology Research and Development Program of Shandong (2018GSF118012), Project of Medical and Health Technology Development Program of Shandong Province (2016WS0268).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ZZ, GL and LY. The first draft of the manuscript was written by ZZ, GL and YJ, all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Research involving human participants and/or animals
The data of this study were drawn from results of the recent large-sample genome-wide association studies on people of European ancestry. Results of these studies belong to public databases. The participants involved in the database have obtained ethical approval. Users can download relevant data for free for research and publish relevant articles. Our study is based on open source data, so there are no ethical issues and other conflicts of interest.
Informed consent
Informed consent of all participants was collected by original researchers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Li, G., Yu, L. et al. Causal relationships between potential risk factors and chronic rhinosinusitis: a bidirectional two-sample Mendelian randomization study. Eur Arch Otorhinolaryngol 280, 2785–2793 (2023). https://doi.org/10.1007/s00405-022-07798-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-022-07798-6