Abstract
Objective
Inguinal lymph node (LN) metastasis is a crucial prognostic factor in vulva carcinoma. The aim of this study was to determine the prognostic value of the number of resected LNs in patients with vulvar carcinoma on recurrence rates.
Methods
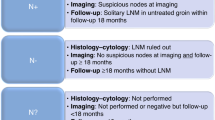
This retrospective study includes patients with vulvar squamous cell carcinoma who underwent inguinofemoral lymphadenectomy (IFL) between 1998 and 2011. Dissected groins were stratified by the number of removed lymph nodes (<6 LNs versus ≥6 LNs) or inguinal LN metastasis (pN− versus pN+) and analyzed according to groin, local and distance recurrence rates.
Results
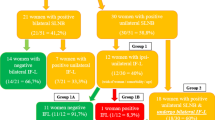
In total 45 patients were identified and 79 groins were eligible for this analysis. 11 patients underwent ipsilateral IFL and 34 bilateral IFL. The median age was 58 years (range 31–80). The median tumor size was 2 cm (range 0.1–7.9). A median of 8 (range 0–19) LNs were resected per groin. Overall in 11 groins LN metastases were found. Groin recurrences occurred in four patients, local recurrence in six patients and distant metastasis in one patient. We did not observe any significant improvement in groin recurrence rates, local recurrence rates and distant recurrence rates if more than six LNs were removed per groin. Notably, patients with LN metastasis did not show higher recurrence rates compared to unaffected LNs.
Conclusion
In this cohort we demonstrated that resection of more than six LNs per groin does not improve the recurrence rates in patients with carcinoma of the vulva. Further prospective studies with more individuals are needed to evaluate the role of resected LNs in vulvar carcinoma.


Similar content being viewed by others
References
Interdisziplinäre S2k-Leitlinie für die Diagnostik und Therapie des Vulvakarzinoms und seiner Vorstufen (2009). 1. Aufl. München, Wien, New York, NY: Zuckschwerdt (Forschen, lehren, heilen)
Jones RW, Baranyai J, Stables S (1997) Trends in squamous cell carcinoma of the vulva: the influence of vulvar intraepithelial neoplasia. Obstet Gynecol 90(3):448–452
Kimmig, Rainer (2001): Vulvakarzinom. [Empfehlungen zur Diagnostik, Therapie und Nachsorge]. 1. Aufl. München [u.a.]: Zuckschwerdt (Manual/Tumorzentrum München an den Medizinischen Fakultäten der Ludwig-Maximilians-Universität und der Technischen Universität)
Hacker NF, Berek JS, Lagasse LD, Leuchter RS, Moore JG (1983) Management of regional lymph nodes and their prognostic influence in vulvar cancer. Obstet Gynecol 61(4):408–412
Beller U, Quinn MA, Benedet JL, Creasman WT, Ngan HYS, Maisonneuve P et al (2006): Carcinoma of the vulva. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 95(Suppl 1):S7–S27
Raspagliesi Francesco, Hanozet Francesco, Ditto Antonino, Solima Eugenio, Zanaboni Flavia, Vecchione Francesca, Kusamura Shigeki (2006) Clinical and pathological prognostic factors in squamous cell carcinoma of the vulva. Gynecol Oncol 102(2):333–337
Ansink A, van der Velden J (2000) Surgical interventions for early squamous cell carcinoma of the vulva. Cochrane Database Syst Rev 2:CD002036
Cormio G, Loizzi V, Carriero C et al (2010) Groin recurrence in carcinoma of the vulva: management and outcome. Eur J Cancer Care (Engl) 19:302Y307
Stehman FB, Bundy BN, Dvoretsky PM, Creasman WT (1992) Early stage I carcinoma of the vulva treated with ipsilateral superficial inguinal lymphadenectomy and modified radical hemivulvectomy: a prospective study of the Gynecologic Oncology Group. Obstet Gynecol 79(4):490–497
Baiocchi G, Cestari FM, Rocha RM, Faloppa CC, Kumagai LY, Fukazawa EM et al (2013) Does the count after inguinofemoral lymphadenectomy in vulvar cancer correlate with outcome? Eur J Surg Oncol 39(4):339–343
Courtney-Brooks Madeleine, Sukumvanich Paniti, Beriwal Sushil, Zorn Kristin K, Richard Scott D, Krivak Thomas C (2010) Does the number of nodes removed impact survival in vulvar cancer patients with node-negative disease? Gynecol Oncol 117(2):308–311
Le Tien, Elsugi Ramadan, Hopkins Laura, Faught Wylam, Fung-Kee-Fung Michael (2007) The definition of optimal inguinal femoral nodal dissection in the management of vulva squamous cell carcinoma. Ann Surg Oncol 14(7):2128–2132
van Beekhuizen HJ, Auzin M, van den Einden LC, de Hullu JA, van der Velden J, Wildhagen MF, van Doorn HC (2014) Lymph node count at inguinofemoral lymphadenectomy and groin recurrences in vulvar cancer. Int J Gynecol Cancer 24(4):773–778
Pecorelli Sergio (2009) Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 105(2):103–104
Likes W, Santoso JT, Wan J (2013) A cross-sectional analysis of lower genital tract intraepithelial neoplasia in immune-compromised women with an abnormal Pap. Arch Gynecol Obstet 287(4):743–747
Wittekind, Christian (Hg.) (2010) TNM—Klassifikation maligner Tumoren. 7. Aufl. [Weinheim]. Wiley, Weinheim
Lavorato-Rocha, AM, de Melo Maia B, Rodrigues IS, Stiepcich MM, Baiocchi G, da Silva Cestari FM et al (2013) Prognostication of vulvar cancer based on p14ARF status: molecular assessment of transcript and protein. Ann Surg Oncol 20(1):31–39. doi:10.1245/s10434-012-2560-7
Butler JS, Milliken DA, Dina R et al (2010) Isolated groin recurrence in vulval squamous cell cancer. The importance of node count. Eur J Gynaecol Oncol 31:510Y513
Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, Mortel R (1993) Prognostic factors for groin node metastasis in squamous cell carcinoma of the vulva (a Gynecologic Oncology Group study). Gynecol Oncol 49(3):279–283
Levenback C, Morris M, Burke TW, Gershenson DM, Wolf JK, Wharton JT (1996) Groin dissection practices among gynecologic oncologists treating early vulvar cancer. Gynecol Oncol 62(1):73–77
Rouzier Roman, Haddad Bassam, Dubernard Gil, Dubois Philippe, Paniel Bernard-Jean (2003) Inguinofemoral dissection for carcinoma of the vulva: effect of modifications of extent and technique on morbidity and survival. J Am Coll Surg 196(3):442–450
Gordinier Mary E, Malpica Anais, Burke Thomas W, Bodurka Diane C, Wolf Judith K, Jhingran Anuja et al (2003) Groin recurrence in patients with vulvar cancer with negative nodes on superficial inguinal lymphadenectomy. Gynecol Oncol 90(3):625–628
Stehman Frederick B, Ali Shamshad, DiSaia Philip J (2009) Node count and groin recurrence in early vulvar cancer: a Gynecologic Oncology Group study. Gynecol Oncol 113(1):52–56
Oonk MH, van Hemel BM, Hollema H, de Hullu JA, Ansink AC, Vergote I, Verheijen RH, Maggioni A, Gaarenstroom KN, Baldwin PJ, van Dorst EB, van der Velden J, Hermans RH, van der Putten HW, Drouin P, Runnebaum IB, Sluiter WJ, van der Zee AG (2010) Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol 1(7):646–652
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors did not receive any funding from the National Institutes of Health (NIH); Wellcome Trust; Howard Hughes Medical Institute (HHMI); and other. The authors declare that they have full control of all primary data and agree to allow the Journal to review their data if requested.
Electronic supplementary material
Below is the link to the electronic supplementary material.
404_2015_3932_MOESM1_ESM.ppt
Supplementary material 1 (PPT 130 kb) Supp. Figure 1: Recurrence rates based on the number of removed lymph nodes per patient: <12 (n=12) or ≥12 (n=21); (a) Groin recurrence (GR). (b) Local recurrence (LR). (c) Distant recurrence (DR).
Rights and permissions
About this article
Cite this article
Diehl, A., Volland, R., Kirn, V. et al. The number of removed lymph nodes by inguinofemoral lymphadenectomy: impact on recurrence rates in patients with vulva carcinoma. Arch Gynecol Obstet 294, 131–136 (2016). https://doi.org/10.1007/s00404-015-3932-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3932-6