Abstract
Purpose
Oxidative stress (OS) is a major concern in idiopathic male infertility as elevated levels of reactive oxygen species (ROS) in the semen is believed to adversely affect sperm functional competence and damage both nuclear and mitochondrial DNA. Therefore, identifying infertile men with seminal OS may be used as a valuable tool in the assessment of male infertility. Semen is a complex mixture of spermatozoa and seminal plasma which is rich in enzymatic and non-enzymatic antioxidants. However, the measurement of ROS in the semen and its application in male infertility assessment is poorly understood.
Methods
The aim of the present study was to compare the significance of ROS measurement in washed and neat semen. The study included 65 infertile men with abnormal semen (SA) parameters, 17 infertile men with normal semen (NS) parameters and 43 fertile controls (FC). ROS levels in both washed and neat semen were measured by luminol-dependent chemiluminescence assay and the values were expressed as 104 RLU per minute per 20 million spermatozoa.
Results
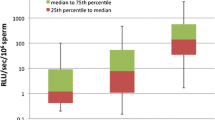
The levels of ROS in both washed and neat semen were found to be significantly higher (P < 0.0001) in infertile men with abnormal and normal semen parameters as compared with FC (P < 0.0001). The lowest median level of ROS was found in FC (neat semen 0.03, washed semen 0.24), whereas infertile men with SA group had the highest median ROS level (neat semen 3.44, washed semen 27.42). In all subjects, ROS levels were found to be higher in washed semen than in neat semen. A strong positive correlation (r = 0.847, P < 0.0001) of ROS levels between neat and washed semen was observed.
Conclusion
Measurement of ROS in neat semen reflects the original oxidative status in the actual sperm microenvironment and is an easy, simple, inexpensive and rapid method compared with ROS measurement from washed semen. ROS measurement in washed semen is done to assess ROS levels following sperm processing as in cases opting for assisted conception. As both ROS values in neat and washed show a positive correlation, neat semen may be used as a valuable tool for identifying infertile men with seminal OS. ROS levels are elevated in nearly 70% men with idiopathic infertility. Hence, ROS analysis in neat semen has both good diagnostic and prognostic value in male infertility assessment.

Similar content being viewed by others
References
Agarwal A, Makker K, Sharma R (2008) Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol 59(1):2–11. doi:10.1111/j.1600-0897.2007.00559.x
Venkatesh S, Riyaz AM, Shamsi MB, Kumar R, Gupta NP, Mittal S, Malhotra N, Sharma RK, Agarwal A, Dada R (2009) Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia 41(4):251–256. doi:10.1111/j.1439-0272.2009.00943.x
Agarwal A, Varghese AC, Sharma RK (2009) Markers of oxidative stress and sperm chromatin integrity. Methods Mol Biol 590:377–402. doi:10.1007/978-1-60327-378-7_24
Ramya T, Misro MM, Sinha D, Nandan D (2010) Sperm function and seminal oxidative stress as tools to identify sperm pathologies in infertile men. Fertil Steril 93(1):297–300. doi:10.1016/j.fertnstert.2009.05.074
Venkatesh S, Deecaraman M, Kumar R, Shamsi MB, Dada R (2009) Role of reactive oxygen species in the pathogenesis of mitochondrial DNA (mtDNA) mutations in male infertility. Indian J Med Res 129(2):127–137
de Lamirande E, Gagnon C (1992) Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl 13(5):368–378
de Lamirande E, Tsai C, Harakat A, Gagnon C (1998) Involvement of reactive oxygen species in human sperm acrosome reaction induced by a23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J Androl 19(5):585–594
Griveau JF, Le Lannou D (1997) Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl 20(2):61–69
Saran M, Bors W (1989) Oxygen radicals acting as chemical messengers: a hypothesis. Free Radic Res Commun 7(3–6):213–220
Shamsi MB, Venkatesh S, Tanwar M, Talwar P, Sharma RK, Dhawan A, Kumar R, Gupta NP, Malhotra N, Singh N, Mittal S, Dada R (2009) DNA integrity and semen quality in men with low seminal antioxidant levels. Mutat Res 665(1–2):29–36. doi:10.1016/j.mrfmmm.2009.02.017
Agarwal A, Nallella KP, Allamaneni SS, Said TM (2004) Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online 8(6):616–627
Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J (2005) Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl 26(3):349–353. doi:10.2164/jandrol.04146
Tunc O, Thompson J, Tremellen K (2009) Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online 18(6):761–768
Agarwal A, Allamaneni SS, Said TM (2004) Chemiluminescence technique for measuring reactive oxygen species. Reprod Biomed Online 9(4):466–468
Kobayashi H, Gil-Guzman E, Mahran AM, Sharma RK, Nelson DR, Thomas AJ Jr, Agarwal A (2001) Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J Androl 22(4):568–574
Shekarriz M, Thomas AJ Jr, Agarwal A (1995) Incidence and level of seminal reactive oxygen species in normal men. Urology 45(1):103–107
Athayde KS, Cocuzza M, Agarwal A, Krajcir N, Lucon AM, Srougi M, Hallak J (2007) Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl 28(4):613–620. doi:10.2164/jandrol.106.001966
Fingerova H, Oborna I, Novotny J, Svobodova M, Brezinova J, Radova L (2009) The measurement of reactive oxygen species in human neat semen and in suspended spermatozoa: a comparison. Reprod Biol Endocrinol 7:118. doi:10.1186/1477-7827-7-118
(1999) WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction, 4th edn. Cambridge University Press, Cambridge
Kumar R, Venkatesh S, Kumar M, Tanwar M, Shasmsi MB, Gupta NP, Sharma RK, Talwar P, Dada R (2009) Oxidative stress and sperm mitochondrial DNA mutation in idiopathic oligoasthenozoospermic men. Indian J Biochem Biophys 46(2):172–177
Mahfouz R, Sharma R, Thiyagarajan A, Kale V, Gupta S, Sabanegh E, Agarwal A (2010) Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil Steril. doi:10.1016/j.fertnstert.2009.12.030
Tunc O, Tremellen K (2009) Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J Assist Reprod Genet 26(9–10):537–544. doi:10.1007/s10815-009-9346-2
Dada R (2010) Recurrent pregnancy loss: male factor. In: Deka D, Malhotra N (eds) An introduction to genetics and fetal medicine. Federation of obstetrics and gynecological society of India publication. Jaypee Brothers, New Delhi, pp 27–37
Epe B, Pflaum M, Haring M, Hegler J, Rudiger H (1993) Use of repair endonucleases to characterize DNA damage induced by reactive oxygen species in cellular and cell-free systems. Toxicol Lett 67(1–3):57–72
Stadtman ER (1993) Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem 62:797–821. doi:10.1146/annurev.bi.62.070193.004053
Von Sonntag C (1994) Topics in free radical-mediated DNA damage: purines and damage amplification-superoxic reactions-bleomycin, the incomplete radiomimetic. Int J Radiat Biol 66(5):485–490
McLeod J (1943) The role of oxygen in the metabolism and motility of human spermatozoa. Am J Physiol 138:512–518
Pasqualotto FF, Sharma RK, Nelson DR, Thomas AJ, Agarwal A (2000) Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril 73(3):459–464
Shen H, Ong C (2000) Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med 28(4):529–536
Hammadeh ME, Radwan M, Al-Hasani S, Micu R, Rosenbaum P, Lorenz M, Schmidt W (2006) Comparison of reactive oxygen species concentration in seminal plasma and semen parameters in partners of pregnant and non-pregnant patients after IVF/ICSI. Reprod Biomed Online 13(5):696–706
Lopes S, Jurisicova A, Sun JG, Casper RF (1998) Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod 13(4):896–900
Saleh RA, Agarwal A, Nelson DR, Nada EA, El-Tonsy MH, Alvarez JG, Thomas AJ Jr, Sharma RK (2002) Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril 78(2):313–318
Derijck AA, van der Heijden GW, Ramos L, Giele M, Kremer JA, de Boer P (2007) Motile human normozoospermic and oligozoospermic semen samples show a difference in double-strand DNA break incidence. Hum Reprod 22(9):2368–2376. doi:10.1093/humrep/dem166
Aziz N, Novotny J, Oborna I, Fingerova H, Brezinova J, Svobodova M (2010) Comparison of chemiluminescence and flow cytometry in the estimation of reactive oxygen and nitrogen species in human semen. Fertil Steril. doi:10.1016/j.fertnstert.2010.03.022
Desai NR, Mahfouz R, Sharma R, Gupta S, Agarwal A (2010) Reactive oxygen species levels are independent of sperm concentration, motility, and abstinence in a normal, healthy, proven fertile man: a longitudinal study. Fertil Steril. doi:10.1016/j.fertnstert.2009.12.041
Agarwal A, Sharma RK, Nallella KP, Thomas AJ Jr, Alvarez JG, Sikka SC (2006) Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril 86(4):878–885. doi:10.1016/j.fertnstert.2006.02.111
Ochsendorf FR, Thiele J, Fuchs J, Schuttau H, Freisleben HJ, Buslau M, Milbradt R (1994) Chemiluminescence in semen of infertile men. Andrologia 26(5):289–293
Aitken RJ, Buckingham D, West K, Wu FC, Zikopoulos K, Richardson DW (1992) Differential contribution of leucocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates of oligozoospermic patients and fertile donors. J Reprod Fertil 94(2):451–462
Venkatesh S, Singh G, Gupta NP, Kumar R, Deecaraman M, Dada R (2009) Correlation of sperm morphology and oxidative stress in infertile men. Iran J Reprod Med 7(1):29–34
Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG (2006) Oxidative stress in an assisted reproductive techniques setting. Fertil Steril 86(3):503–512. doi:10.1016/j.fertnstert.2006.02.088
Acknowledgments
The authors thank All India Institute of Medical Sciences (AIIMS) and Indian Council of Medical research (ICMR) for their financial support. S. Venkatesh is a senior research fellow awarded by ICMR.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkatesh, S., Shamsi, M.B., Dudeja, S. et al. Reactive oxygen species measurement in neat and washed semen: comparative analysis and its significance in male infertility assessment. Arch Gynecol Obstet 283, 121–126 (2011). https://doi.org/10.1007/s00404-010-1645-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1645-4