Abstract
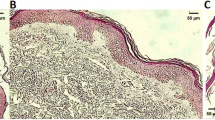
Tuberous sclerosis complex (TSC) is a disorder of cell lineage, migration, proliferation and differentiation, characterized by the development of widespread benign hamartomas, which are particularly evident in hamartomatous lesions of the skin. The aim of this study was to investigate differences in gene expression of certain proteoglycans (PGs) and to characterize glycosaminoglycans (GAGs) in tissue specimens of normal skin, fibrous plaques and angiofibromas from patients with TSC. The expression of PG mRNA was determined by semiquantitative RT-PCR analysis. Total GAGs were isolated from tissue specimens after lipid extraction and extensive digestion with Pronase and DNase and characterized by treatment with GAG-degrading enzymes followed by electrophoresis on polyacrylamide gradient gels and cellulose acetate membranes. Normal skin specimens express versican, decorin and aggrecan and contain hyaluronic acid and dermatan sulphate. In angiofibroma specimens aggrecan is not expressed while versican splice variant with two EGF-like domains and decorin are downregulated. Furthermore, angiofibromas differ from normal skin in that they additionally contain keratan, heparan and chondroitin sulphates and do not contain dermatan sulphate. In fibrous plaque specimens gene expression of PGs was similar to that in normal skin, but with respect to GAGs, they contained a single acidic glycan population that did not share common structural features with known GAGs. The variations of the above ECM molecules between normal and TSC skin may be attributed to TSC-related mutations and, overall, support the TSC-associated pathological manifestations of cell migration, proliferation and differentiation.






Similar content being viewed by others
References
Gomez M, Sampson J, Whittemore V (1999) The tuberous sclerosis complex. Oxford University Press, Oxford
Webb DW, Clarke A, Fryer A, Osborne JP (1996) The cutaneous features of tuberous sclerosis: a population study. Br J Dermatol 135:1–5
Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, Kasprzyk-Obara J, Domanska-Pakiela D, Kwiatkowski DJ (2001) Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 68:64–80
Curatolo P, Verdecchia M, Bombardieri R (2002) Tuberous sclerosis complex: a review of neurological aspects. Eur J Paediatr Neurol 6:15–23
Arbiser JL, Brat D, Hunter S, D'Armiento J, Henske EP, Arbiser ZK, Bai X, Goldberg G, Cohen C, Weiss SW (2002) Tuberous sclerosis-associated lesions of the kidney, brain, and skin are angiogenic neoplasms. J Am Acad Dermatol 46:376–380
Sogut A, Ozmen M, Sencer S, Caliskan M, Aydinli N, Ertugrul T, Peksayar G (2002) Clinical features of tuberous sclerosis cases. Turk J Pediatr 44:98–101
van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JBM, Ward S, Green AJ, Yates JRW, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277:805–808
Gao X, Pan D (2001) TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev 15:1383–1392
Potter CJ, Huang H, Xu T (2001) Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell 105:357–368
Siegel DH, Howard R (2002) Molecular advances in genetic skin diseases. Curr Opin Pediatr 14:419–425
Lallier TE (1991) Cell lineage and cell migration in the neural crest. Ann N Y Acad Sci 615:158–171
Papakonstantinou E, Karakiulakis G, Eickelberg O, Perruchoud AP, Block LH, Roth M (1998) A 340 kDa hyaluronic acid secreted by human vascular smooth muscle cells regulates their proliferation and migration. Glycobiology 8:821–830
Passi A, Albertini R, Campagnari F, De Luca G (1997) Modifications of proteoglycans secreted into the growth medium by young and senescent human skin fibroblasts. FEBS Lett 402:286–290
Edwards IJ, Wagner ED (1988) Distinct synthetic and structural characteristics of proteoglycans produced by cultured artery smooth muscle cells of atherosclerosis-susceptible pigeons. J Biol Chem 263:9612–9620
McQuillan DJ, Yanagishita M, Hascall V, Bickel M (1989) Proteoglycan biosynthesis in murine monocytic leukemic (MI) cells before and after differentiation. J Biol Chem 264:13245–13251
Terry DE, Clark AF (1996) Glycosaminoglycans in the three lobes of the rat prostate following castration and testosterone treatment. Biochem Cell Biol 74:653–658
Khalkhali-Ellis Z, Henderson K, Hemming FW (1991) Glycoprotein and proteoglycan alterations in tuberous sclerosis. Ann N Y Acad Sci 615:149–157
Uysal H, Saxton J, Hemming FW (1997) Changes in the secretion and glycosylation of fibronectin by human skin fibroblasts associated with tuberous sclerosis. Glycoconj J 14:439–447
Uysal H, Hemming FW (1999) Changes in the expression and distribution of fibronectin, laminin and tenascin by cultured fibroblasts from skin lesions of patients with tuberous sclerosis. Br J Dermatol 141:658–666
Fischer MH, Fortune JS, Foster SH, Gilbert EF (1977) Chemical analysis of an angiofibroma from a patient with tuberous sclerosis. J Ment Defic Res 21:251–261
Oikarinen A, Palatsi R, Linna SL, Peltonen L (1982) Types I and III collagens and the activities of prolyl hydroxylase and galactosylhydroxylysyl glucosyltransferase in skin lesions of tuberous sclerosis. Br J Dermatol 107:659–664
Kjellen L, Lindahl U (1991) Proteoglycans: structures and interactions. Annu Rev Biochem 60:443–475
Iozzo RV (1999) The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem 274:18843–18846
Iozzo RV (1998) Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 67:609–652
Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV (1995) De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci U S A 92:7016–7020
De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV (1996) Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem 271:18961–18965
Mogyorosi A, Ziyadeh FN (1998) Increased decorin mRNA in diabetic mouse kidney and in mesangial and tubular cells cultured in high glucose. Am J Physiol 275:F827–F832
Nishimura M, Yan W, Mukudai Y, Nakamura S, Nakamasu K, Kawata M, Kawamoto T, Noshiro M, Hamada T, Kato Y (1998) Role of chondroitin sulfate-hyaluronan interactions in the viscoelastic properties of extracellular matrices and fluids. Biochim Biophys Acta 1380:1–9
Papakonstantinou E, Roth M, Block LH, Mirtsou-Fidani V, Argiriadis P, Karakiulakis G (1998) The differential distribution of hyaluronic acid in the layers of human atheromatic aortas is associated with vascular smooth muscle cell proliferation and invasion. Atherosclerosis 138:79–89
Bitter T, Muir H (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330–334
Papakonstantinou E, Roth M, Tamm M, Eickelberg O, Perruchoud AP, Karakiulakis G (2002) Hypoxia differentially enhances the effects of transforming growth factor-β isoforms on the synthesis and secretion of glycosaminoglycans by human lung fibroblasts. J Pharmacol Exp Ther 301:830–837
Papakonstantinou E, Karakiulakis G, Tamm M, Perruchoud AP, Roth M (2000) Hypoxia modifies the effect of PDGF on glycosaminoglycan synthesis by primary human lung cells. Am J Physiol 279:L825–L834
Papakonstantinou E, Misevic GN (1993) Isolation and characterization of a new class of acidic glycans implicated in sea urchin embryonal cell adhesion. J Cell Biochem 53:98–113
Grover J, Roughley PJ (1993) Versican gene expression in human articular cartilage and comparison of mRNA splicing variation with aggrecan. Biochem J 291:361–367
Krusius T, Ruoslahti E (1986) Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A 83:7683–7687
Doege KJ, Sasaki M, Kimura T, Yamada Y (1991) Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem 266:894–902
Roberts PS, Chung J, Jozwiak S, Dabora SL, Franz DN, Thiele EA, Kwiatkowski DJ (2002) SNP identification, haplotype analysis, and parental origin of mutations in TSC2. Hum Genet 111:96–101
Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV (1997) Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility, J Cell Biol 136:729–743
Scott JE (1996) Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry 35:8795–8799
Rollins BJ, Culp LA (1979) Glycosaminoglycans in the substrate adhesion sites of normal and virus transformed murine cells. Biochemistry 18:141–148
Zimmermann DR, Dours-Zimmermann MT, Schubert M, Bruckner-Tuderman L (1994) Versican is expressed in the proliferation zone in the epidermis and in association with the elastic network of the dermis. J Cell Biol 124:817–825
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papakonstantinou, E., Dionyssopoulos, A., Pesintzaki, C. et al. Expression of proteoglycans and glycosaminoglycans in angiofibroma and fibrous plaque skin lesions from patients with tuberous sclerosis. Arch Dermatol Res 295, 138–145 (2003). https://doi.org/10.1007/s00403-003-0413-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-003-0413-8