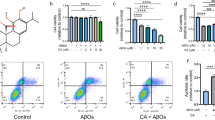
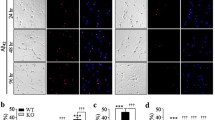
Abstract
Soluble oligomers of amyloid-β (Aβ) impair synaptic plasticity, perturb neuronal energy homeostasis, and are implicated in Alzheimer’s disease (AD) pathogenesis. Therefore, significant efforts in AD drug discovery research aim to prevent the formation of Aβ oligomers or block their neurotoxicity. The eukaryotic elongation factor-2 kinase (eEF2K) plays a critical role in synaptic plasticity, and couples neurotransmission to local dendritic mRNA translation. Recent evidence indicates that Aβ oligomers activate neuronal eEF2K, suggesting a potential link to Aβ induced synaptic dysfunction. However, a detailed understanding of the role of eEF2K in AD pathogenesis, and therapeutic potential of eEF2K inhibition in AD, remain to be determined. Here, we show that eEF2K activity is increased in postmortem AD patient cortex and hippocampus, and in the hippocampus of aged transgenic AD mice. Furthermore, eEF2K inhibition using pharmacological or genetic approaches prevented the toxic effects of Aβ42 oligomers on neuronal viability and dendrite formation in vitro. We also report that eEF2K inhibition promotes the nuclear factor erythroid 2-related factor (NRF2) antioxidant response in neuronal cells, which was crucial for the beneficial effects of eEF2K inhibition in neurons exposed to Aβ42 oligomers. Accordingly, NRF2 knockdown or overexpression of the NRF2 inhibitor, Kelch-Like ECH-Associated Protein-1 (Keap1), significantly attenuated the neuroprotection associated with eEF2K inhibition. Finally, genetic deletion of the eEF2K ortholog efk-1 reduced oxidative stress, and improved chemotaxis and serotonin sensitivity in C. elegans expressing human Aβ42 in neurons. Taken together, these findings highlight the potential utility of eEF2K inhibition to reduce Aβ-mediated oxidative stress in AD.








Similar content being viewed by others
References
Alonso-Nanclares L, Merino-Serrais P, Gonzalez S, DeFelipe J (2013) Synaptic changes in the dentate gyrus of APP/PS1 transgenic mice revealed by electron microscopy. J Neuropathol Exp Neurol 72:386–395. doi:10.1097/NEN.0b013e31828d41ec
Baxter PS, Bell KF, Hasel P, Kaindl AM, Fricker M, Thomson D et al (2015) Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat Commun 6:6761. doi:10.1038/ncomms7761
Bender CL, Yang Q, Sun L, Liu SJ (2016) NH125 reduces the level of CPEB3, an RNA binding protein, to promote synaptic GluA2 expression. Neuropharmacology 101:531–537. doi:10.1016/j.neuropharm.2015.03.017
Boyce M, Py BF, Ryazanov AG, Minden JS, Long K, Ma D et al (2008) A pharmacoproteomic approach implicates eukaryotic elongation factor 2 kinase in ER stress-induced cell death. Cell Death Differ 15:589–599. doi:10.1038/sj.cdd.4402296
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Bryan HK, Olayanju A, Goldring CE, Park BK (2013) The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol 85:705–717. doi:10.1016/j.bcp.2012.11.016
Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B et al (2012) Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 109:8740–8745. doi:10.1073/pnas.1206171109
Carrera I, Etcheverria I, Li Y, Fernandez-Novoa L, Lombardi V, Vigo C et al (2013) Immunocytochemical characterization of Alzheimer disease hallmarks in APP/PS1 transgenic mice treated with a new anti-amyloid-beta vaccine. Biomed Res Int 2013:709145. doi:10.1155/2013/709145
Chen Z, Gopalakrishnan SM, Bui MH, Soni NB, Warrior U, Johnson EF et al (2011) 1-Benzyl-3-cetyl-2-methylimidazolium iodide (NH125) induces phosphorylation of eukaryotic elongation factor-2 (eEF2): a cautionary note on the anticancer mechanism of an eEF2 kinase inhibitor. J Biol Chem 286:43951–43958. doi:10.1074/jbc.M111.301291
Chu HP, Liao Y, Novak JS, Hu Z, Merkin JJ, Shymkiv Y et al (2014) Germline quality control: eEF2K stands guard to eliminate defective oocytes. Dev Cell 28:561–572. doi:10.1016/j.devcel.2014.01.027
Chuang TT (2010) Neurogenesis in mouse models of Alzheimer’s disease. Biochim Biophys Acta 1802:872–880. doi:10.1016/j.bbadis.2009.12.008
De Strooper B, Karran E (2016) The cellular phase of Alzheimer’s disease. Cell 164:603–615. doi:10.1016/j.cell.2015.12.056
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8:101–112. doi:10.1038/nrm2101
Habas A, Hahn J, Wang X, Margeta M (2013) Neuronal activity regulates astrocytic Nrf2 signaling. Proc Natl Acad Sci USA 110:18291–18296. doi:10.1073/pnas.1208764110
Hamilton A, Vasefi M, Vander Tuin C, McQuaid RJ, Anisman H, Ferguson SS (2016) Chronic pharmacological mGluR5 inhibition prevents cognitive impairment and reduces pathogenesis in an alzheimer disease mouse model. Cell Rep 15:1859–1865. doi:10.1016/j.celrep.2016.04.077
Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N (2002) MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol 158:541–549. doi:10.1083/jcb.200110134
Harder B, Jiang T, Wu T, Tao S, de la Vega MR, Tian W et al (2015) Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem Soc Trans 43:680–686. doi:10.1042/BST20150020
Heise C, Gardoni F, Culotta L, di Luca M, Verpelli C, Sala C (2014) Elongation factor-2 phosphorylation in dendrites and the regulation of dendritic mRNA translation in neurons. Front Cell Neurosci 8:35. doi:10.3389/fncel.2014.00035
Hobert O (2003) Behavioral plasticity in C. elegans: paradigms, circuits, genes. J Neurobiol 54:203–223. doi:10.1002/neu.10168
Jan A, Adolfsson O, Allaman I, Buccarello AL, Magistretti PJ, Pfeifer A et al (2010) A{beta}42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete A{beta}42 species. J Biol Chem. doi:10.1074/jbc.M110.172411
Jan A, Hartley DM, Lashuel HA (2010) Preparation and characterization of toxic A[beta] aggregates for structural and functional studies in Alzheimer’s disease research. Nat Protocols 5:1186–1209. http://www.nature.com/nprot/journal/v5/n6/suppinfo/nprot.2010.72_S1.html
Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA et al (2004) Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13:159–170
Johnson DA, Johnson JA (2015) Nrf2–a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med 88:253–267. doi:10.1016/j.freeradbiomed.2015.07.147
Keith D, El-Husseini A (2008) Excitation control: balancing PSD-95 function at the synapse. Front Mol Neurosci 1:4. doi:10.3389/neuro.02.004.2008
Kenney JW, Moore CE, Wang X, Proud CG (2014) Eukaryotic elongation factor 2 kinase, an unusual enzyme with multiple roles. Adv Biol Regul 55:15–27. doi:10.1016/j.jbior.2014.04.003
Kessels HW, Nabavi S, Malinow R (2013) Metabotropic NMDA receptor function is required for beta-amyloid-induced synaptic depression. Proc Natl Acad Sci USA 110:4033–4038. doi:10.1073/pnas.1219605110
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M et al (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95:6448–6453
Lansbury PT, Lashuel HA (2006) A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature 443:774–779. doi:10.1038/nature05290
Leprivier G, Remke M, Rotblat B, Dubuc A, Mateo AR, Kool M et al (2013) The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell 153:1064–1079. doi:10.1016/j.cell.2013.04.055
LeVine H 3rd, Walker LC (2016) What amyloid ligands can tell us about molecular polymorphism and disease. Neurobiol Aging 42:205–212. doi:10.1016/j.neurobiolaging.2016.03.019
Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D (2009) Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62:788–801. doi:10.1016/j.neuron.2009.05.012
Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ (2005) Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. FEBS J 272:4211–4220. doi:10.1111/j.1742-4658.2005.04833.x
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. doi:10.1038/nature05292
Liu R, Proud CG (2016) Eukaryotic elongation factor 2 kinase as a drug target in cancer, and in cardiovascular and neurodegenerative diseases. Acta Pharmacol Sin 37:285–294. doi:10.1038/aps.2015.123
Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y et al (2014) REST and stress resistance in ageing and Alzheimer’s disease. Nature 507:448–454. doi:10.1038/nature13163
Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Marambaud P et al (2014) Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid beta. J Neurosci 34:12230–12238. doi:10.1523/JNEUROSCI.1694-14.2014
Manzoni C, Colombo L, Bigini P, Diana V, Cagnotto A, Messa M et al (2011) The molecular assembly of amyloid abeta controls its neurotoxicity and binding to cellular proteins. PLoS One 6:e24909. doi:10.1371/journal.pone.0024909
Margie O, Palmer C, Chin-Sang I (2013) C. elegans chemotaxis assay. J Vis Exp e50069. doi:10.3791/50069
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702. doi:10.1016/j.neuron.2011.05.001
Mitsuishi Y, Motohashi H, Yamamoto M (2012) The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2:200. doi:10.3389/fonc.2012.00200
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R et al. (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39:409–421. doi:S0896627303004343 [pii]
Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA et al (2007) Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol 66:75–85. doi:10.1097/nen.0b013e31802d6da9
Roselli F, Tirard M, Lu J, Hutzler P, Lamberti P, Livrea P et al (2005) Soluble beta-amyloid1-40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. JNeurosci 25:11061–11070
Rotblat B, Southwell AL, Ehrnhoefer DE, Skotte NH, Metzler M, Franciosi S et al (2014) HACE1 reduces oxidative stress and mutant Huntingtin toxicity by promoting the NRF2 response. Proc Natl Acad Sci USA 111:3032–3037. doi:10.1073/pnas.1314421111
Ryazanov AG (2002) Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett 514:26–29. doi:10.1016/S0014-5793(02)02299-8
Sakamoto S, Ishii K, Sasaki M, Hosaka K, Mori T, Matsui M et al (2002) Differences in cerebral metabolic impairment between early and late onset types of Alzheimer’s disease. J Neurol Sci 200:27–32. doi:10.1016/S0022-510X(02)00114-4
Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M (2011) AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem 118:460–474. doi:10.1111/j.1471-4159.2011.07331.x
Saraiva LM, Seixas da Silva GS, Galina A, da-Silva WS, da-Silva WS, Klein WL, Ferreira ST et al (2010) Amyloid-beta triggers the release of neuronal hexokinase 1 from mitochondria. PLoS One 5:e15230. doi:10.1371/journal.pone.0015230
Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MP et al (2005) Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis 18:602–617. doi:10.1016/j.nbd.2004.10.022
Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26:619–631. doi:10.1016/S0896-6273(00)81199-X
Scheetz AJ, Nairn AC, Constantine-Paton M (2000) NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci 3:211–216. doi:10.1038/72915
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81:741–766
Selkoe DJ (2008) Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res 192:106–113. doi:10.1016/j.bbr.2008.02.016
Selkoe DJ (2013) The therapeutics of Alzheimer’s disease: where we stand and where we are heading. Ann Neurol 74:328–336. doi:10.1002/ana.24001
Selkoe DJ, Schenk D (2003) Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol 43:545–584
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189. doi:10.1101/cshperspect.a006189
Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM (2007) Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron 55:648–661. doi:10.1016/j.neuron.2007.07.030
Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Tremblay RG, Sikorska M, Sandhu JK, Lanthier P, Ribecco-Lutkiewicz M, Bani-Yaghoub M (2010) Differentiation of mouse Neuro 2A cells into dopamine neurons. J Neurosci Methods 186:60–67. doi:10.1016/j.jneumeth.2009.11.004
Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H et al (2013) Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron 79:887–902. doi:10.1016/j.neuron.2013.06.036
Vandal M, White PJ, Tournissac M, Tremblay C, St-Amour I, Drouin-Ouellet J et al (2016) Impaired thermoregulation and beneficial effects of thermoneutrality in the 3xTg-AD model of Alzheimer’s disease. Neurobiol Aging 43:47–57. doi:10.1016/j.neurobiolaging.2016.03.024
Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA (2008) Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci 28:13574–13581. doi:10.1523/JNEUROSCI.4099-08.2008
Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A et al (1999) Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. JBiolChem 274:25945–25952
Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B et al (2002) Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res 924:133–140. doi:10.1016/S0006-8993(01)03058-X
Wilcock DM, Gordon MN, Morgan D (2006) Quantification of cerebral amyloid angiopathy and parenchymal amyloid plaques with Congo red histochemical stain. Nat Protoc 1:1591–1595. doi:10.1038/nprot.2006.277
Wisniewski T, Goni F (2015) Immunotherapeutic approaches for Alzheimer’s disease. Neuron 85:1162–1176. doi:10.1016/j.neuron.2014.12.064
Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL et al (2006) Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci 26:13102–13113. doi:10.1523/JNEUROSCI.3448-06.2006
Xie H, Hou S, Jiang J, Sekutowicz M, Kelly J, Bacskai BJ (2013) Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proc Natl Acad Sci USA 110:7904–7909. doi:10.1073/pnas.1217938110
Acknowledgments
This work was supported by the Ride2Survive Brain Cancer Impact Grant of the Canadian Cancer Society and Brain Canada (grant #703205), and funds from the BC Cancer Foundation, to PHS, a postdoctoral fellowship from the Canadian Institutes of Health Research to AJ, and a Tier 2 Canadian Research Chair in Transcriptional Regulatory Networks to ST. The authors would like to thank Amy Li, Haifeng Zhang, Jordan Cran, Arash Samiei, and David Ko for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jan, A., Jansonius, B., Delaidelli, A. et al. eEF2K inhibition blocks Aβ42 neurotoxicity by promoting an NRF2 antioxidant response. Acta Neuropathol 133, 101–119 (2017). https://doi.org/10.1007/s00401-016-1634-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-016-1634-1