Abstract
In multiple sclerosis (MS), the immune cell attack leads to axonal injury as a major cause for neurological disability. Here, we report a novel role of the cell adhesion molecule L1 in the crosstalk between the immune and nervous systems. L1 was found to be expressed by CNS axons of MS patients and human T cells. In MOG35–55-induced murine experimental neuroinflammation, CD4+ T cells were associated with degenerating axons in the spinal cord, both expressing L1. However, neuronal L1 expression in the spinal cord was reduced, while levels of the transcriptional repressor REST (RE1-Silencing Transcription Factor) were up-regulated. In PLP139–151-induced relapsing–remitting neuroinflammation, L1 expression was low at the peak stage of disease, reached almost normal levels in the remission stage, but decreased again during disease relapse indicating adaptive expression regulation of L1. In vitro, activated CD4+ T cells caused contact-dependent down-regulation of L1, up-regulation of its repressor REST and axonal injury in co-cultured neurons. T cell adhesion to neurons and axonal injury were prevented by an antibody blocking L1 suggesting that down-regulation of L1 ameliorates neuroinflammation. In support of this hypothesis, antibody-mediated blocking of L1 in C57BL/6 mice as well as neuron-specific depletion of L1 in synapsinCre × L1fl/fl mice reduces disease severity and axonal pathology despite unchanged immune cell infiltration of the CNS. Our data suggest that down-regulation of neuronal L1 expression is an adaptive process of neuronal self-defense in response to pro-inflammatory T cells, thereby alleviating immune-mediated axonal injury.








Similar content being viewed by others
References
Appel F, Holm J, Conscience JF, von Bohlen F, Faissner A, James P, Schachner M (1995) Identification of the border between fibronectin type III homologous repeats 2 and 3 of the neural cell adhesion molecule L1 as a neurite outgrowth promoting and signal transducing domain. J Neurobiol 28:297–312
Balaian LB, Moehler T, Montgomery AMP (2000) The human neural cell adhesion molecule L1 functions as a costimulatory molecule in T cell activation. Eur J Immunol 30:938–943
Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, Abouzid K, Guan J-L, Tessier-Lavigne M, Lemmon V, Castellani V (2008) FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J 27:1549–1562
Ben-Nun A, Kaushansky N, Kawakami N, Krishnamoorthy G, Berer K, Liblau R, Hohlfeld R, Wekerle H (2014) From classic to spontaneous and humanized models of multiple sclerosis: Impact on understanding pathogenesis and drug development. J Autoimmun 54:33–50
Ben-Zvi A, Manor O, Schachner M, Yaron A, Tessier-Lavigne M, Behar O (2008) The semaphorin receptor plexinA3 mediates neuronal apoptosis during dorsal root ganglia development. J Neurosci 28:12427–12432
Brewer GJ, Price PJ (1996) Viable cultured neurons in ambient carbon dioxide and hibernation storage for a month. NeuroReport 7:1509–1512
Calderone A, Jover T, K-m Noh, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R, Bennett MVL, Zukin RS (2003) Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci 23:2112–2121
Castellani V, De Angelis E, Kenwrick S, Rougon G (2002) Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J 21:6348–6357
Christaller WA, Vos Y, Gebre-Medhin S, Hofstra RM, Schafer MK (2016) L1 syndrome diagnosis complemented with functional analysis of L1CAM variants located to the two N-terminal Ig-like domains. Clin Genet. doi:10.1111/cge.12763
Cohen NR, Taylor JSH, Scott LB, Guillery RW, Soriano P, Furley AJW (1998) Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol 8:26–33
Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N (1997) Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet 17:346–349
De Gasperi R, Rocher AB, Sosa MAG, Wearne SL, Perez GM, Friedrich VL, Hof PR, Elder GA (2008) The IRG mouse: a two-color fluorescent reporter for assessing Cre-mediated recombination and imaging complex cellular relationships in situ. Genesis 46:308–317
Derfuss T, Parikh K, Velhin S, Braun M, Mathey E, Krumbholz M, Kümpfel T, Moldenhauer A, Rader C, Sonderegger P, Pöllmann W, Tiefenthaller C, Bauer J, Lassmann H, Wekerle H, Karagogeos D, Hohlfeld R, Linington C, Meinl E (2009) Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc Natl Acad Sci USA 106:8302–8307
Ebeling O, Duczmal A, Aigner S, Geiger C, Schöllhammer S, Kemshead JT, Möller P, Schwartz-Albiez R, Altevogt P (1996) L1 adhesion molecule on human lymphocytes and monocytes: expression and involvement in binding to αvβ3 integrin. Eur J Immunol 26:2508–2516
Formisano L, Guida N, Laudati G, Boscia F, Esposito A, Secondo A, Di Renzo G, Canzoniero LM (2015) Extracellular signal-related kinase 2/specificity protein 1/specificity protein 3/repressor element-1 silencing transcription factor pathway is involved in Aroclor 1254-induced toxicity in SH-SY5Y neuronal cells. J Neurosci Res 93:167–177
Fransen E, D’Hooge R, Van Camp G, Verhoye M, Sijbers J, Reyniers E, Soriano P, Kamiguchi H, Willemsen R, Koekkoek SKE, De Zeeuw CI, De Deyn PP, Van der Linden A, Lemmon V, Kooy RF, Willems PJ (1998) L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Hum Mol Genet 7:999–1009
Irintchev A, Schachner M (2012) The injured and regenerating nervous system: immunoglobulin superfamily members as key players. Neuroscientist 18:452–466
Islam R, Kristiansen LV, Romani S, Garcia-Alonso L, Hortsch M (2004) Activation of EGF receptor kinase by L1-mediated homophilic cell interactions. Mol Biol Cell 15:2003–2012
Jakeman LB, Chen Y, Lucin KM, McTigue DM (2006) Mice lacking L1 cell adhesion molecule have deficits in locomotion and exhibit enhanced corticospinal tract sprouting following mild contusion injury to the spinal cord. Eur J Neurosci 23:1997–2011
Jakovcevski I, Djogo N, Hölters LS, Szpotowicz E, Schachner M (2013) Transgenic overexpression of the cell adhesion molecule L1 in neurons facilitates recovery after mouse spinal cord injury. Neuroscience 252:1–12
Jolivel V, Luessi F, Masri J, Kraus SHP, Hubo M, Poisa-Beiro L, Klebow S, Paterka M, Yogev N, Tumani H, Furlan R, Siffrin V, Jonuleit H, Zipp F, Waisman A (2013) Modulation of dendritic cell properties by laquinimod as a mechanism for modulating multiple sclerosis. Brain 136:1048–1066. doi:10.1093/brain/awt023
Jonas A, Thiem S, Kuhlmann T, Wagener R, Aszodi A, Nowell C, Hagemeier K, Laverick L, Perreau V, Jokubaitis V, Emery B, Kilpatrick T, Butzkueven H, Gresle M (2014) Axonally derived matrilin-2 induces proinflammatory responses that exacerbate autoimmune neuroinflammation. J Clin Invest 124:5042–5056
Jouet M, Rosenthal A, Kenwrick S (1995) Exon 2 of the gene for neural cell adhesion molecule L1 is alternatively spliced in B cells. Brain Res Mol Brain Res 30:378–380
Kadmon G, Montgomery AMP, Altevogt P (1998) L1 makes immunological progress by expanding its relations. Dev Immunol 6:205–213. doi:10.1155/1998/23451
Kallunki P, Edelman GM, Jones FS (1997) Tissue-specific expression of the L1 cell adhesion molecule is modulated by the neural restrictive silencer element. J Cell Biol 138:1343–1354. doi:10.1083/jcb.138.6.1343
Kataria H, Lutz D, Chaudhary H, Schachner M, Loers G (2015) Small molecule agonists of cell adhesion molecule L1 mimic L1 functions in vivo. Mol Neurobiol 1–23. doi:10.1007/s12035-015-9352-6
Larionov A, Krause A, Miller W (2005) A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6:62. doi:10.1186/1471-2105-6-62
Law JWS, Lee AYW, Sun M, Nikonenko AG, Chung SK, Dityatev A, Schachner M, Morellini F (2003) Decreased anxiety, altered place learning, and increased CA1 basal excitatory synaptic transmission in mice with conditional ablation of the neural cell adhesion molecule L1. J Neurosci 23:10419–10432
Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, Shirvan A, Barzilai A, Hermine O (2006) Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol 36:1782–1793
Leuenberger T, Paterka M, Reuter E, Herz J, Niesner RA, Radbruch H, Bopp T, Zipp F, Siffrin V (2013) The role of CD8 + T cells and their local interaction with CD4 + T cells in myelin oligodendrocyte glycoprotein35–55-induced experimental autoimmune encephalomyelitis. J Immunol 191:4960–4968. doi:10.4049/jimmunol.1300822
Liblau RS, Gonzalez-Dunia D, Wiendl H, Zipp F (2013) Neurons as targets for T cells in the nervous system. Trends Neurosci 36:315–324
Maddaluno L, Verbrugge SE, Martinoli C, Matteoli G, Chiavelli A, Zeng Y, Williams ED, Rescigno M, Cavallaro U (2009) The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J Exp Med 206:623–635
Maness PF, Schachner M (2007) Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci 10:19–26
Marx M, Diestel S, Bozon M, Keglowich L, Drouot N, Bouché E, Frebourg T, Minz M, Saugier-Veber P, Castellani V, Schäfer M (2012) Pathomechanistic characterization of two exonic L1CAM variants located in trans in an obligate carrier of X-linked hydrocephalus. Neurogenetics 13:49–59
Mathey EK, Derfuss T, Storch MK, Williams KR, Hales K, Woolley DR, Al-Hayani A, Davies SN, Rasband MN, Olsson T, Moldenhauer A, Velhin S, Hohlfeld R, Meinl E, Linington C (2007) Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med 204:2363–2372. doi:10.1084/jem.20071053
Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, Riedle S, Postina R, Fahrenholz F, Fogel M, Lemmon V, Altevogt P (2001) Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol 155:661–674
Miller NM, Shriver LP, Bodiga VL, Ray A, Basu S, Ahuja R, Jana A, Pahan K, Dittel BN (2013) Lymphocytes with cytotoxic activity induce rapid microtubule axonal destabilization independently and before signs of neuronal death. ASN Neuro 5
Nesti E, Corson GM, McCleskey M, Oyer JA, Mandel G (2014) C-terminal domain small phosphatase 1 and MAP kinase reciprocally control REST stability and neuronal differentiation. Proc Natl Acad Sci USA 111:2
Nijland PG, Michailidou I, Witte ME, Mizee MR, van der Pol SM, van Het Hof B, Reijerkerk A, Pellerin L, van der Valk P, de Vries HE, van Horssen J (2014) Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia 62:1125–1141. doi:10.1002/glia.22667
Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Bruck W, Bishop D, Misgeld T, Kerschensteiner M (2011) A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 17:495–499
Paquette AJ, Perez SE, Anderson DJ (2000) Constitutive expression of the neuron-restrictive silencer factor (NRSF)/REST in differentiating neurons disrupts neuronal gene expression and causes axon pathfinding errors in vivo. Proc Natl Acad Sci USA 97:12318–12323
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45
Pozzi D, Lignani G, Ferrea E, Contestabile A, Paonessa F, D’Alessandro R, Lippiello P, Boido D, Fassio A, Meldolesi J, Valtorta F, Benfenati F, Baldelli P (2013) REST/NRSF-mediated intrinsic homeostasis protects neuronal networks from hyperexcitability. EMBO J 32:2994–3007
Rathjen FG, Schachner M (1984) Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J 3:1–10
Roonprapunt C, Huang W, Grill R, Friedlander D, Grumet M, Chen S, Schachner M, Young W (2003) Soluble cell adhesion molecule L1-Fc promotes locomotor recovery in rats after spinal cord injury. J Neurotrauma 20:871–882
Runker AE, Bartsch U, Nave K-A, Schachner M (2003) The C264Y missense mutation in the extracellular domain of L1 impairs protein trafficking in vitro and in vivo. J Neurosci 23:277–286
Ruppert M, Aigner S, Hubbe M, Yagita H, Altevogt P (1995) The L1 adhesion molecule is a cellular ligand for VLA-5. J Cell Biol 131:1881–1891
Saghatelyan AK, Nikonenko AG, Sun M, Rolf B, Putthoff P, Kutsche M, Bartsch U, Dityatev A, Schachner M (2004) Reduced GABAergic transmission and number of hippocampal perisomatic inhibitory synapses in juvenile mice deficient in the neural cell adhesion molecule L1. Mol Cell Neurosci 26:191–203
Sawma P, Roth L, Blanchard C, Bagnard D, Cremel G, Bouveret E, Duneau JP, Sturgis JN, Hubert P (2014) Evidence for new homotypic and heterotypic interactions between transmembrane helices of proteins involved in receptor tyrosine kinase and neuropilin signaling. J Mol Biol 426:4099–4111
Schäfer M, Altevogt P (2010) L1CAM malfunction in the nervous system and human carcinomas. Cell Mol Life Sci 67:2425–2437
Schäfer MKE, Frotscher M (2012) Role of L1CAM for axon sprouting and branching. Cell Tissue Res 349:39–48
Schmid J, Bernreuther C, Nikonenko A, Ling Z, Mies G, Hossmann K-A, Jakovcevski I, Schachner M (2013) Heterozygosity for the mutated X-chromosome-linked L1 cell adhesion molecule gene leads to increased numbers of neurons and enhanced metabolism in the forebrain of female carrier mice. Brain Struct Funct 218:1375–1390
Shriver LP, Dittel BN (2006) T-cell-mediated disruption of the neuronal microtubule network: correlation with early reversible axonal dysfunction in acute experimental autoimmune encephalomyelitis. Am J Pathol 169:999–1011
Siffrin V, Brandt AU, Radbruch H, Herz J, Boldakowa N, Leuenberger T, Werr J, Hahner A, Schulze-Topphoff U, Nitsch R, Zipp F (2009) Differential immune cell dynamics in the CNS cause CD4 + T cell compartmentalization. Brain 132:1247–1258
Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, Laube G, Luche H, Lehnardt S, Fehling H-J, Griesbeck O, Zipp F (2010) In Vivo imaging of partially reversible Th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 33:424–436
Siffrin V, Vogt J, Radbruch H, Nitsch R, Zipp F (2010) Multiple sclerosis—candidate mechanisms underlying CNS atrophy. Trends Neurosci 33:202–210
Sorbara Catherine D, Wagner Naomi E, Ladwig A, Nikić I, Merkler D, Kleele T, Marinković P, Naumann R, Godinho L, Bareyre Florence M, Bishop D, Misgeld T, Kerschensteiner M (2014) Pervasive axonal transport deficits in multiple sclerosis models. Neuron 84:1183–1190
Takeda Y, Asou H, Murakami Y, Miura M, Kobayashi M, Uyemura K (1996) A nonneuronal isoform of cell adhesion molecule L1: tissue-specific expression and functional analysis. J Neurochem 66:2338–2349
Tanabe S, Yamashita T (2014) Repulsive guidance molecule-a is involved in Th17-cell-induced neurodegeneration in autoimmune encephalomyelitis. Cell Rep 9:1459–1470
Trapp BD, Nave KA (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 31:247–269
Tsenter J, Beni-Adani L, Assaf Y, Alexandrovich AG, Trembovler V, Shohami E (2008) Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J Neurotrauma 25:324–333
Wehner AB, Abdesselem H, Dickendesher TL, Imai F, Yoshida Y, Giger RJ, Pierchala BA (2016) Semaphorin 3A is a retrograde cell death signal in developing sympathetic neurons. Development 143:1560–1570
Williams A, Piaton G, Aigrot M-S, Belhadi A, Théaudin M, Petermann F, Thomas J-L, Zalc B, Lubetzki C (2007) Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain 130:2554–2565
Zhao Y, Zhu M, Yu Y, Qiu L, Zhang Y, He L, Zhang J (2016) Brain REST/NRSF is not only a silent repressor but also an active protector. Mol Neurobiol 7:7
Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF (2001) Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Gene Dev 15:859–876
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft, CRC1080 Project B6 to MKES and FZ. The anti-L1 antibody clones 555 and 324 were developed in the laboratory of Melitta Schachner and a generous gift from Peter Altevogt (DKFZ, Heidelberg, Germany). We gratefully acknowledge the technical assistance of Tobias Hirnet, Christine Oswald and Heike Ehrengard and proofreading of the manuscript by Dr. Cheryl Ernest (all UMC Mainz, Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Frauke Zipp and Michael K. E. Schäfer are equally contributing co-senior authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2016_1607_MOESM1_ESM.tif
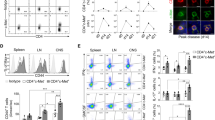
Supplemental Fig. S1. CD4+ T cell subsets express L1 and cause reduction of neuronal L1 mRNA expression. (a) Flow cytometry showing similar L1 expression on CD4+ T cell subsets isolated from the CNS of C57BL/6J mice at the peak of disease upon MOG35-55-induced EAE (n=6). (b) Neuronal L1 mRNA expression is reduced upon 2 h of co-culturing with CD4+ T cell subsets, differentiated from spleen-derived CD4+ T cells (n=6/subset, normalized to βIII-tubulin and control, set as 1, which represents neurons cultured alone). Data are expressed as mean ± SEM. βIII-tubulin immunofluorescence is normalized to control conditions which represent neurons cultured alone (set as 100 %). Generation of CD4+ T cell subpopulations: Antigen-presenting cells (APC) from spleen of 8-10-week-old C57BL6/J mice were enriched by T cell depletion using the CD90+ MicroBeads kit (Miltenyi), irradiated (30 Gy) and cultured in full culture medium (10 % FCS, 0,01 % β-mercaptoethanol, 1 % glutamate, 1 % penicillin/streptomycin, 1 % HEPES). Naïve T cells were isolated using the CD4+CD62L+ MicroBeads kit (Miltenyi) and cultured with APCs and anti-CD3 antibodies (2 µg/mL). Differentiation of CD4 subpopulations was stimulated using subset-specific cytokine combinations (all cytokines purchased from R&D Systems) and respective antibodies (all Bio X cell) as followed: Th1 cells (IL-12, 50 ng/ml; IL-18, 25 ng/ml; anti-IL-4 antibody, 10 µg/ml), Th2 cells (IL-4, 10 ng/ml; anti-IL-12 antibody, 10 µg/mL; anti-IFNγ antibody, 10 µg/mL), Th17 cells (IL-23, 20 ng/mL; IL-6, 20 ng/mL; TGFβ, 3 ng/mL; anti-IL-4 antibody, 10 µg/mL; anti-IFNγ antibody, 10 µg/mL), regulatory T cells (TGFβ, 3 ng/mL; anti-IL-12 antibody, 10 µg/mL; anti-IFNγ antibody, 10 µg/mL). Th1, Th2 and regulatory T cells were used after 5 days of differentiation, Th17 cells were re-stimulated for additional 5 days before use. All subpopulations were routinely tested for subset-specific cytokine expression using intracellular staining and flow cytometry analysis (data not shown). (TIFF 1741 kb)
401_2016_1607_MOESM2_ESM.tif
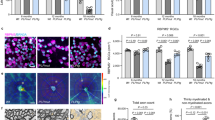
Supplemental Fig. S2. Axonal loss in spinal cord tissue. (a) Representative image showing a spinal cord hemisphere of C57BL/6J mice at the peak of disease, immunolabeled with antibodies specific to the axonal marker neurofilament (anti-NF) and counterstained with DAPI to visualize cell nuclei. Boxes indicate regions with high and low cell densities. (b, c) High-power magnifications showing NF/DAPI-double labeling. The number of axons is strongly reduced in tissue regions with high cell densities. Arrows depict NF-labeled axons. Scale: 200 µm (a), 20 µm (b). (TIFF 3800 kb)
Rights and permissions
About this article
Cite this article
Menzel, L., Paterka, M., Bittner, S. et al. Down-regulation of neuronal L1 cell adhesion molecule expression alleviates inflammatory neuronal injury. Acta Neuropathol 132, 703–720 (2016). https://doi.org/10.1007/s00401-016-1607-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-016-1607-4