Abstract
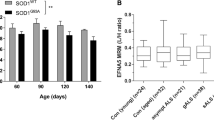
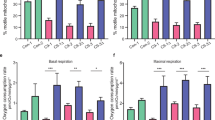
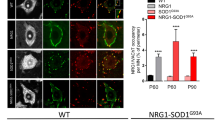
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease with unknown origins. Neurodegeneration in ALS mouse models occurs together with signs of disrupted blood–spinal cord barrier (BSCB) and regressed capillary network, but the molecular pathways contributing to these vascular pathologies remain unknown. We show that motor neurons of human sporadic ALS patients (n = 12) have increased gene expression of PDGFC and its activator PLAT and presymptomatic activation of the PDGF-CC pathway in SOD1 G93A mice leads to BSCB dysfunction. Decrease of Pdgfc expression in SOD1 G93A mice restored vascular barrier properties, reduced motor neuron loss and delayed symptom onset by up to 3 weeks. Similarly, lower expression levels of PDGFC or PLAT in motor neurons of sporadic ALS patients were correlated with older age at disease onset. PDGF-CC inhibition and restoration of BSCB integrity did not prevent capillary regression at disease end stage. Lower vessel density was found in spinal cords of sporadic ALS patients and the degree of regression in SOD1 G93A mice correlated with more aggressive progression after onset regardless of BSCB status. We conclude that PDGF-CC-induced BSCB dysfunction can contribute to timing of ALS onset, allow insight into disease origins and development of targeted novel therapies.



Similar content being viewed by others
References
Abrams MB, Nilsson I, Lewandowski SA, Kjell J, Codeluppi S, Olson L, Eriksson U (2012) Imatinib enhances functional outcome after spinal cord injury. PLoS One 7:e38760. doi:10.1371/journal.pone.0038760
Adzemovic MV, Zeitelhofer M, Eriksson U, Olsson T, Nilsson I (2013) Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response. PLoS One 8:e56586. doi:10.1371/journal.pone.0056586
Al-Chalabi A, Hardiman O (2013) The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 9:617–628. doi:10.1038/nrneurol.2013.203
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C (2010) Pericytes regulate the blood-brain barrier. Nature 468:557–561. doi:10.1038/nature09522
Beers DR, Ho BK, Siklos L, Alexianu ME, Mosier DR, Mohamed AH, Otsuka Y, Kozovska ME, McAlhany RE, Smith RG, Appel SH (2001) Parvalbumin overexpression alters immune-mediated increases in intracellular calcium, and delays disease onset in a transgenic model of familial amyotrophic lateral sclerosis. J Neurochem 79:499–509
Bento-Abreu A, Van Damme P, Van Den Bosch L, Robberecht W (2010) The neurobiology of amyotrophic lateral sclerosis. Eur J Neurosci 31:2247–2265. doi:10.1111/j.1460-9568.2010.07260.x
Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW (2006) Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312:1389–1392. doi:10.1126/science.1123511
Brown WR (2010) A review of string vessels or collapsed, empty basement membrane tubes. J Alzheimers Dis 21:725–739. doi:10.3233/JAD-2010-100219
Ding H, Wu X, Bostrom H, Kim I, Wong N, Tsoi B, O’Rourke M, Koh GY, Soriano P, Betsholtz C, Hart TC, Marazita ML, Field LL, Tam PP, Nagy A (2004) A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet 36:1111–1116. doi:10.1038/ng1415
Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD (2004) Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 185:232–240. doi:10.1016/j.expneurol.2003.10.004
Fredriksson L, Li H, Fieber C, Li X, Eriksson U (2004) Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J 23:3793–3802. doi:10.1038/sj.emboj.7600397
Fredriksson L, Nilsson I, Su EJ, Andrae J, Ding H, Betsholtz C, Eriksson U, Lawrence DA (2012) Platelet-derived growth factor C deficiency in C57BL/6 mice leads to abnormal cerebral vascularization, loss of neuroependymal integrity, and ventricular abnormalities. Am J Pathol 180:1136–1144. doi:10.1016/j.ajpath.2011.12.006
Fredriksson L, Stevenson TK, Su EJ, Ragsdale M, Moore S, Craciun S, Schielke GP, Murphy GG, Lawrence DA (2015) Identification of a neurovascular signaling pathway regulating seizures in mice. Ann Clin Transl Neurol 2:722–738. doi:10.1002/acn3.209
Fruttiger M, Calver AR, Richardson WD (2000) Platelet-derived growth factor is constitutively secreted from neuronal cell bodies but not from axons. Curr Biol 10:1283–1286. doi:10.1016/S0960-9822(00)00757-0
Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR (2007) Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res 1157:126–137. doi:10.1016/j.brainres.2007.04.044
Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, Lin CL (2003) Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet 12:2519–2532. doi:10.1093/hmg/ddg267
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX et al (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264:1772–1775
Johnston CA, Stanton BR, Turner MR, Gray R, Blunt AH, Butt D, Ampong MA, Shaw CE, Leigh PN, Al-Chalabi A (2006) Amyotrophic lateral sclerosis in an urban setting: a population based study of inner city London. J Neurol 253:1642–1643. doi:10.1007/s00415-006-0195-y
Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E (2010) CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci 123:1073–1080. doi:10.1242/jcs.059329
Leitner M, Menzies S, Lutz C (2010) Working with ALS Mice. Guidelines for preclinical testing and colony management. Prize4Life and The Jackson Laboratory, pp 1–21. http://www.researchals.org/uploaded_files/p4l_jax_sod1manual_20091202_29aPcx.pdf
Leonardi A, Abbruzzese G, Arata L, Cocito L, Vische M (1984) Cerebrospinal fluid (CSF) findings in amyotrophic lateral sclerosis. J Neurol 231:75–78
Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E (2008) Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol 183:409–417. doi:10.1083/jcb.200806024
Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, Scalettar BA (2006) Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol 66:564–577. doi:10.1002/neu.20250
Ludolph AC, Bendotti C, Blaugrund E, Hengerer B, Loffler JP, Martin J, Meininger V, Meyer T, Moussaoui S, Robberecht W, Scott S, Silani V, Van Den Berg LH (2007) Guidelines for the preclinical in vivo evaluation of pharmacological active drugs for ALS/MND: report on the 142nd ENMC international workshop. Amyotroph Lateral Scler 8:217–223. doi:10.1080/17482960701292837
Mae M, Armulik A, Betsholtz C (2011) Getting to know the cast–cellular interactions and signaling at the neurovascular unit. Curr Pharm Des 17:2750–2754. doi:10.2174/13816121179744011
Miyazaki K, Masamoto K, Morimoto N, Kurata T, Mimoto T, Obata T, Kanno I, Abe K (2011) Early and progressive impairment of spinal blood flow–glucose metabolism coupling in motor neuron degeneration of ALS model mice. J Cereb Blood Flow Metab 32:456–467. doi:10.1038/jcbfm.2011.155
Neuwelt EA (2004) Mechanisms of disease: the blood–brain barrier. Neurosurgery 54:131–140. doi:10.1227/01.NEU.0000097715.11966.8E
Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O’Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR (2011) Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 12:169–182. doi:10.1038/nrn2995
Prudencio M, Belzil VV, Batra R, Ross CA, Gendron TF, Pregent LJ, Murray ME, Overstreet KK, Piazza-Johnston AE, Desaro P, Bieniek KF, DeTure M, Lee WC, Biendarra SM, Davis MD, Baker MC, Perkerson RB, van Blitterswijk M, Stetler CT, Rademakers R, Link CD, Dickson DW, Boylan KB, Li H, Petrucelli L (2015) Distinct brain transcriptome profiles in C9orf72-associated and sporadic ALS. Nat Neurosci 18:1175–1182. doi:10.1038/nn.4065
Rabin SJ, Kim JM, Baughn M, Libby RT, Kim YJ, Fan Y, La Spada A, Stone B, Ravits J (2010) Sporadic ALS has compartment-specific aberrant exon splicing and altered cell-matrix adhesion biology. Hum Mol Genet 19:313–328. doi:10.1093/hmg/ddp498
Regal L, Vanopdenbosch L, Tilkin P, Van den Bosch L, Thijs V, Sciot R, Robberecht W (2006) The G93C mutation in superoxide dismutase 1: clinicopathologic phenotype and prognosis. Arch Neurol 63:262–267. doi:10.1001/archneur.63.2.262
Rosen DR (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 364:362. doi:10.1038/364362c0
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62. doi:10.1038/362059a0
Rule RR, Schuff N, Miller RG, Weiner MW (2010) Gray matter perfusion correlates with disease severity in ALS. Neurology 74:821–827. doi:10.1212/WNL.0b013e3181d3e2dd
Seelen M, van Doormaal PT, Visser AE, Huisman MH, Roozekrans MH, de Jong SW, van der Kooi AJ, de Visser M, Voermans NC, Veldink JH, van den Berg LH (2014) Prior medical conditions and the risk of amyotrophic lateral sclerosis. J Neurol 261:1949–1956. doi:10.1007/s00415-014-7445-1
Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA (2008) Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med 14:731–737. doi:10.1038/nm1787
Su EJ, Fredriksson L, Kanzawa M, Moore S, Folestad E, Stevenson TK, Nilsson I, Sashindranath M, Schielke GP, Warnock M, Ragsdale M, Mann K, Lawrence AE, Medcalf RL, Eriksson U, Murphy GG, Lawrence DA (2015) Imatinib treatment reduces brain injury in a murine model of traumatic brain injury. Front Cell Neurosci 9:385. doi:10.3389/fncel.2015.00385
Van Laere K, Vanhee A, Verschueren J, De Coster L, Driesen A, Dupont P, Robberecht W, Van Damme P (2014) Value of 18fluorodeoxyglucose-positron-emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol. doi:10.1001/jamaneurol.2014.62
Wang W, Salvaterra PM, Loera S, Chiu AY (1997) Brain-derived neurotrophic factor spares choline acetyltransferase mRNA following axotomy of motor neurons in vivo. J Neurosci Res 47:134–143. doi:10.1002/(SICI)1097-4547(19970115)47:2<134:AID-JNR2>3.0.CO;2-G
Weydt P, Hong SY, Kliot M, Moller T (2003) Assessing disease onset and progression in the SOD1 mouse model of ALS. NeuroReport 14:1051–1054. doi:10.1097/01.wnr.0000073685.00308.89
Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z, Sullivan JS, Griffin JH, Cleveland DW, Zlokovic BV (2014) Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci USA. doi:10.1073/pnas.1401595111
Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV (2012) Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 125:111–120. doi:10.1007/s00401-012-1039-8
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11:251–253. doi:10.1038/nn2047
Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV (2008) ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci 11:420–422. doi:10.1038/nn2073
Acknowledgments
This work was supported by grants from the Thierry Latran Foundation (U. E.), the Leducq Foundation (U. E.), Swedish Research Council (U.E. 2011-3861), the Swedish Governmental Agency for Innovation Systems (VINNOVA, U. E., L. F. 2011-03503), the Ragnar Söderberg Foundation (E. H. M245/11), the Swedish Brain Foundation (U. E., E. H. FO2012-0055) and the Hållsten Research Foundation (U. E.), the Birgit Backmark Donation (E. H.), the Åhlen’s Foundation (E. H. mA9/11, mA5/h12 and mB8/h13), the Swedish Stroke Foundation (I. N.), the Swedish Research Council (I. N. 524-2008-785, L. F. 524-2008-777 and 521-2012-1853), the National Institutes of Health (D. A. L. R01 NS079639) and Karolinska Institutet. Human post mortem tissues were provided by the Netherland’s Brain Bank (NBB). We would like to thank Sofia Wittgren and Mark Warnock for technical assistance. We would also like to thank R. K. Filipkowski for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Video 1 (WMV 24231 kb)
Rights and permissions
About this article
Cite this article
Lewandowski, S.A., Nilsson, I., Fredriksson, L. et al. Presymptomatic activation of the PDGF-CC pathway accelerates onset of ALS neurodegeneration. Acta Neuropathol 131, 453–464 (2016). https://doi.org/10.1007/s00401-015-1520-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1520-2