Abstract
For nearly a century, the diagnosis and grading of oligodendrogliomas and oligoastrocytomas has been based on histopathology alone. Roughly 20 years ago, the first glioma-associated molecular signature was found with complete chromosome 1p and 19q codeletion being particularly common in histologically classic oligodendrogliomas. Subsequently, this codeletion appeared to not only carry diagnostic, but also prognostic and predictive information, the latter aspect only recently resolved after carefully constructed clinical trials with very long follow-up times. More recently described biomarkers, including the non-balanced translocation leading to 1p/19q codeletion, promoter hypermethylation of the MGMT gene, mutations of the IDH1 or IDH2 gene, and mutations of FUBP1 (on 1p) or CIC (on 19q), have greatly enhanced our understanding of oligodendroglioma biology, although their diagnostic, prognostic, and predictive roles are less clear. It has therefore been suggested that complete 1p/19q codeletion be required for the diagnosis of ‘canonical oligodendroglioma’. This transition to an integrated morphological and molecular diagnosis may result in the disappearance of oligoastrocytoma as an entity, but brings new challenges as well. For instance it needs to be sorted out how (histopathological) criteria for grading of ‘canonical oligodendrogliomas’ should be adapted, how pediatric oligodendrogliomas (known to lack codeletions) should be defined, which platforms and cut-off levels should ideally be used for demonstration of particular molecular aberrations, and how the diagnosis of oligodendroglioma should be made in centers/countries where molecular diagnostics is not available. Meanwhile, smart integration of morphological and molecular information will lead to recognition of biologically much more uniform groups within the spectrum of diffuse gliomas and thereby facilitate tailored treatments for individual patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For over a century, classification of neoplasms has been based on their microscopic characteristics and the histopathological diagnosis has provided the cornerstone for therapeutic decision-making. The ‘taxonomy’ of tumors of the central nervous system (CNS) in the most recent, i.e., 2007 World Health Organization (WHO) classification of these neoplasms is still largely based on their histopathological features as well [73]. Diffuse gliomas are by far the most frequent neoplasms originating from the CNS parenchyma itself. The common denominator of this heterogeneous group of neoplasms is extensive, infiltrative growth of individual tumor cells and/or groups of these cells (‘diffuse growth’) into the neuropil, i.e., the dense network of neuronal and glial processes of the CNS parenchyma [23].
Over 75 % of the diffuse gliomas in adult patients are astrocytic, about two-thirds of these being represented by the most malignant form, glioblastoma. Oligodendrogliomas and oligoastrocytomas have been generally grouped together as oligodendroglial tumors and account for less than 10 % of the diffuse gliomas (Fig. 1). Of note, in children, glioma variants with a more circumscribed growth pattern are more frequent than diffuse gliomas, and oligodendroglial tumors are rare (<4 % of the primary CNS tumors) [85]. According to the WHO 2007 classification, after typing a diffuse glioma as astrocytic, oligodendroglial, or mixed, these neoplasms are graded as WHO grade II/low grade, WHO grade III/anaplastic or WHO grade IV/glioblastoma (WHO grade I being reserved for more circumscribed gliomas, such as pilocytic astrocytoma). Microscopic features used for grading are the presence/absence of marked mitotic activity, florid microvascular proliferation (MVP, i.e., marked hypertrophy and hyperplasia of endothelial cells and pericytes resulting in a multilayered aspect of the microvessel walls), and necrosis [73]. Unequivocal histological typing and grading of diffuse gliomas is challenging however, especially because the biological diversity of these tumors is difficult to capture in precise microscopic criteria. Also, the samples provided for histopathological analysis are not always (fully) representative. This results in a high rate of interobserver variation in the diagnosis of diffuse gliomas, including oligodendroglial tumors [36, 65, 117].
Relative frequency of histopathologically diagnosed oligodendroglial and oligoastrocytic tumors in the spectrum of glial tumors of the CNS: a in 95,564 patients of all age groups; b in 10,274 children and adolescents (0–19 years). Information extracted from CBTRUS statistical report: NPCR and SEER, 2007–2011 [85]
For the past 20 years, the knowledge on the genetic/molecular underpinnings of gliomagenesis is rapidly expanding. It is now fully clear that certain molecular aberrations carry important diagnostic, prognostic and/or predictive information because they provide clinically relevant information on tumor type, biological behavior, and/or expected response to a particular treatment regimen, respectively. It is therefore to be expected that (like, e.g., sarcomas and hematological malignancies) classification of CNS tumors will increasingly be based on the presence of particular molecular aberrations, and oligodendroglial tumors are amongst the first in line for such a change.
In this review, we reconstruct the transition from a purely morphological to an integrated morphological and molecular scheme for classification of the heterogeneous group of oligodendroglial tumors. After summarizing clinical, radiological and histopathological aspects of these neoplasms (‘Setting the stage’), the molecular underpinnings of oligodendroglial tumors and the diagnostic, prognostic and/or predictive value of these aberrations will be discussed (‘Molecular mechanisms and markers’). In the last part of this article (‘WHO’s next?’), we elaborate on how these markers can be used to reclassify oligodendroglial tumors more objectively, but with some challenges and unanswered questions remaining. For a review on similar aspects concerning diffuse astrocytomas, glioblastomas, and pilocytic astrocytomas the reader is referred to companion articles in the present cluster.
Setting the stage
Gliomas originate from neural stem cells or glial progenitor cells that develop or maintain glial characteristics [136]. According to the WHO 2007 classification, based on the resemblance of the tumor cells with non-neoplastic glial cells most diffuse gliomas can be typed as astrocytic, oligodendroglial, or mixed (i.e., oligoastrocytoma) [73]. Oligodendroglial tumors most commonly arise in the frontal lobe, somewhat more frequently in males and with the peak incidence in the 5th and 6th decade. Robust enhancement is not a common feature in low-grade examples and suggests transformation to a higher histologic grade [61, 73]. Most oligodendroglioma patients present with seizures. Patients experiencing generalized tonic–clonic seizures were reported to more often have the greatest lesion load in mesial frontal regions, including cortex connected to the genu of the corpus callosum, while in patients with partial seizures, the oligodendrogliomas were located more caudo-laterally in orbitofrontal and temporal lobes but typically sparing cortex connected to the genu, indicating that the genu of the corpus callosum is a major pathway for seizure generalization [130].
Because of their locally aggressive behavior and the fact that they cannot be cured by current therapies, diffuse gliomas are considered one of the most devastating cancers [23]. Progress has been made with oligodendroglioma patients such that current standard of care (surgery, radiotherapy and chemotherapy) yields median survival times of 12–14 years even in WHO grade III examples. Patients with a WHO grade II oligodendroglioma may survive even longer, but sooner or later such tumors generally progress to a high-grade malignant glioma as well. Given the often young age at presentation, however, this patient category still substantially contributes to the high average number of ‘years of life lost’ for patients suffering from a CNS tumor [17]. Rarely, extracranial metastases of oligodendroglial tumors occur (for recent reviews, see [71, 78]). Only a tiny fraction of oligodendroglial tumors is thought to be due to single-gene hereditary syndromes such as biallelic Lynch syndrome [37, 42].
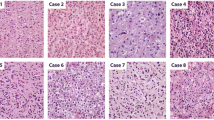
As originally described by Bailey and Bucy [7], nuclear features are key for microscopic recognition of an oligodendroglial phenotype, although some architectural growth patterns also provide useful clues (Fig. 2). In low-grade oligodendrogliomas, the nuclei are generally round and uniform with crisp nuclear membranes, delicate chromatin and small-to-inconspicuous nucleoli. In anaplastic examples, cells are frequently enlarged and epithelioid with nuclei that often show increased size and pleomorphism, a more vesicular chromatin pattern and more prominent nucleoli, but the tumor still maintains an overall sense of regularity and nuclear roundness [80, 81]. The clear, perinuclear halo that often is seen in oligodendroglial tumor cells is in fact an artifact of formalin fixation, which is typically absent in frozen sections and rapidly fixed specimens. The combination of the round nuclei and perinuclear haloes results in a fried-egg appearance of individual cells and in a honeycomb architectural pattern of groups of evenly spaced cells.
Common histopathologic patterns encountered in classic (IDH mutant, 1p19q codeleted) oligodendrogliomas include: a a honeycomb-like arrangement of evenly spaced tumor cells with uniformly rounded nuclei and clear haloes imparting a “fried egg” appearance; additionally, there are branching capillaries (arrow) resembling chicken-wire; b mucin-filled microcystic spaces; c, d minigemistocytes with small rounded bellies of eosinophilic cytoplasm and classic nuclear cytology including rounded countours, delicate “salt and pepper” chromatin, sharp nuclear membranes, and small nucleoli (c intra-operative smear; d tissue section); e foci of anaplastic transformation with increased cellularity and enlarged cell size (left) in comparison to the low-grade precursor cells (right). Anaplastic examples often feature: f enlarged epithelioid cells with increased pleomorphism, vesicular chromatin, macronucleoli, but a maintained general sense of nuclear roundness; g increased mitotic index and occasional “red crunchy” cells with brightly eosinophilic lysosomal inclusions (arrow); h microvascular proliferation; and i foci of tumor necrosis, more often consisting of collections of apoptotic cells rather than the pattern of pseudopalisading necrosis seen in glioblastomas. Immunohistochemically, some cases are completely GFAP negative, with only reactive astrocytes staining (j), while others (k) show strong expression in gliofibrillary oligodendrocytes (perinuclear rim and variable tadpole-like tail) or minigemistocytes (not shown). l A stain for neurofilament protein often highlights the infiltrative growth pattern by showing entrapped neurons and axons. m Tumoral synaptophysin positivity is not uncommon and can include a paranuclear dot-like pattern. n A stain for IDH1-R132H mutant protein highlights perineuronal satellitosis in this positive tumor with cortical involvement. Unlike diffuse astrocytomas, most oligodendrogliomas show only limited p53 immunoreactivity (o) and retained ATRX expression (p)
Oligodendroglial tumors are often highly cellular lesions in central regions with closely packed, relatively small cells. However, in less cellular areas at the tumor periphery, the diffuse infiltrative growth is easily appreciated, often with prominent secondary structure formation, such as clustering of tumor cells around the perikarya of preexistent neurons (satellitosis), under the pial surface (subpial aggregation), and surrounding cortical small vessels (perivascular aggregates). The presence of a branching network of delicate capillaries (chicken-wire pattern) and extensive calcification are common, but not specific additional features in oligodendrogliomas. Rare oligodendroglial tumors show marked pleomorphism (‘polymorphic oligodendroglioma’) or cells arranged in a rhythmic/spongioblastic fashion. Furthermore, part of the tumor cells in oligodendrogliomas may show a gliofibrillary or minigemistocytic phenotype with strong staining for glial fibrillary acidic protein (GFAP) of the cytoplasm, or rarely even signet-ring cell morphology [68, 69, 89]. Typically, oligodendrogliomas in children are histologically similar, but may involve unusual sites such as the posterior fossa and the spinal cord more frequently and lack the molecular background of adult counterparts [102]. Occasionally, a mostly pediatric, slowly progressive oligodendroglial-like proliferation presents as a diffuse leptomeningeal neoplasm [97, 100]. The latter may include the 1p19q codeletion pattern of adult oligodendroglioma, but more frequently harbors 1p deletion without 19q loss [79, 101].
Histopathological typing of diffuse gliomas is relatively straightforward for tumors at the ‘prototypic ends’. However, there is a surprisingly wide morphological spectrum that includes both common and diagnostically misleading patterns (Figs. 2, 3). As indicated by their name, the histopathological diagnosis of oligoastrocytomas is based on the presence of neoplastic glial cells with morphological characteristics of both astrocytes and oligodendrocytes. These cells may be diffusely mixed or separated, although the latter form is rare [73]. The diagnostic definitions of oligoastrocytomas have been subjective and their diagnosis is poorly reproducible. In fact, conceptual preferences of individual (neuro)pathologists further impact cell type determinations. For instance, those who readily accept the existence of mixed gliomas are likely to more liberally diagnose oligoastrocytomas than those skeptical that this entity exists (Fig. 4a) [16, 35].
Less common and sometimes diagnostically confusing histopathologic patterns encountered in classic (IDH mutant, 1p19q codeleted) oligodendrogliomas include: a–e a lobulated or nodular growth pattern can simulate a neuroendocrine tumor, with immunohistochemical pitfalls including CD56 (b) and synaptophysin (c) positivity; however, demonstration of IDH1 R132H mutant protein (d) and 1p19q codeletion confirmed the diagnosis in this case; e focal spindled cytology was also encountered, simulating a more astrocytoma-like pattern. Rare examples demonstrate more overt neuronal differentiation, including neurocytic (f) and ganglioglioma-like (g, h). Neurocytic foci often feature slightly smaller and more hyperchromatic nuclei surrounding central collections of delicate pink neuropil (i.e., neurocytic rosettes). In contrast, the case with ganglioglioma-like maturation featured areas of classic anaplastic oligodendroglioma (g note also the “red crunchy” cells); FISH studies (not shown) revealed 1p19q codeletions not only in this component, but in neoplastic ganglion cells (h) as well
Diffuse gliomas: from histopathologically to molecularly defined entities. Diffuse gliomas histopathologically form a spectrum, both with regard to cell type (astrocytic, oligodendroglial, mixed) and malignancy grade. Especially, delineation of oligoastrocytomas from (more) pure astrocytic and oligodendroglial tumors is poorly reproducible. In practice, (neuro)pathologists who readily accept the existence of mixed gliomas will more liberally diagnose oligoastrocytomas, while those who are skeptical that this entity exists will designate the vast majority of diffuse gliomas as either astrocytic or oligodendroglial (a). Introducing 1p/19q codeletion as a defining feature for oligodendrogliomas will in most cases allow for a clear distinction from astrocytic neoplasms and can be expected to drastically reduce the fraction of neoplasms diagnosed as mixed/oligoastrocytic (b). Meanwhile, especially in children, CNS tumors resembling classic oligodendrogliomas often lack 1p/19q codeletion; the best position for these ‘pediatric oligodendrogliomas’ in an improved taxonomy of CNS tumors requires further study. c Molecular markers helpful in daily clinical practice for state-of-the-art classification of diffuse gliomas. A recently published algorithm proposes immunohistochemistry (IHC) as a first step, allowing for correct classification of esp. the IDH1 R132H mutant protein positive, ATRX negative (and thus ATRX mutated) astrocytomas [96]. Ideally, in other tumors, subsequent molecular testing for 1p/19q codeletion status and/or other IDH1 and IDH2 mutations is performed. Variable testing for other markers can be helpful as well: in IDH mutated (mut) diffuse gliomas demonstration of TERT mutation indicates oligodendroglioma and of TP53 mutation astrocytoma; demonstration of TERT mutation in IDH wild type (wt) diffuse gliomas as well as of EGFR amplification/EGFRvIII strongly indicates high-grade malignant astrocytic tumor/glioblastoma; a KIAA-BRAF fusion gene is typically found in pilocytic astrocytoma, while mutations in the histone genes H3F3A, HIST1H3B/C indicate pediatric type high-grade gliomas (which may also harbor ATRX mutations and show loss of ATRX immunoreactivity). A, O, OA low-grade astrocytoma, oligodendroglioma, oligoastrocytic/mixed glioma, resp.; AA, AO, AOA anaplastic astrocytoma, oligodendroglioma, oligoastrocytic/mixed glioma, resp.; GBM glioblastoma, GBM-O glioblastoma with oligodendroglial component. See also text in this article and the other reviews in this cluster
While in the past, different systems for the assessment of malignancy grade of oligodendroglial tumors were applied [67], the WHO classification has since gained world-wide acceptance [60, 73]. According to the WHO criteria, an oligodendroglioma without necrosis, florid MVP, or marked mitotic activity is considered as WHO grade II, while the presence of one or more of these features generally warrants an anaplastic designation (WHO grade III). Nevertheless, considerable problems arise in daily practice given the subjectivities of these definitions. For instance, how many mitoses are needed for “marked mitotic activity”? One study reported that finding six or more mitoses per ten high power fields allowed for recognition of oligodendrogliomas with more aggressive (WHO grade III) clinical behavior [36]. Additional challenges arise because the grading criteria for astrocytomas differ and as such, the initial determination of astrocytoma versus oligodendroglioma is often critical to whether the same glioma will be considered low- or high-grade.
In oligodendrogliomas, florid MVP and necrosis do not have the same unfavorable connotation as in diffuse astrocytic neoplasms, and such tumors are still considered WHO grade III. In mixed gliomas, the presence of florid MVP was similarly considered to be consistent with WHO grade III, whereas prior to the 2007 WHO classification, the significance of necrosis was unclear. In a large study published in 2006, necrosis was found to be a statistically significant predictor of poor overall survival for patients with anaplastic oligoastrocytoma, but not for those with anaplastic oligodendroglioma [80]. Others published similar observations [107, 119], leading to the conclusion that for anaplastic oligoastrocytomas, stratification into grade III and IV on the basis of absence or presence of necrosis was justified leading to a ‘glioblastoma with oligodendroglial component’ (GBM-O) category in the WHO 2007 classification [73]. Of note, the concept of GBM-O had already been around for more than a decade [24].
More recently, Appin et al. [4] reported that GBM-Os tend to arise in younger patients, more frequently originate from lower grade precursors and are associated with longer survival. The better prognosis of patients with GBM-O compared to those with classic glioblastoma in this series may then at least partly be explained by the fact that these GBM-Os were more often secondary glioblastomas with IDH mutation occurring in younger patients with dedifferentiated tumors [56], a clinical and molecular subtype already known for a better prognosis. Other studies on GBM-O provided conflicting results (see for review [29]). For example, of 339 glioblastoma cases included in the EORTC_26981/NCIC_CE.3 trial and originally diagnosed as glioblastoma by a local pathologist, 15 % was reclassified as GBM-O but this re-grouping lacked prognostic significance [44]. Acknowledging that histopathological distinction of GBM-O from small cell glioblastoma can be difficult, it is likely that the latter variant was overrepresented in some studies taking glioblastomas as a starting point, particularly given their reportedly high frequencies of EGFR gene amplification. These data once again illustrate the fact that histopathological discrimination of mixed gliomas from pure counterparts remains subjective and at least partly explain differences in overall survival of patients with anaplastic oligoastrocytomas between various large prospective studies.
Not infrequently, other primary CNS tumors may show oligodendroglioma-like features including fried-egg appearance of (part of) the tumor cells. Examples are glial neoplasms like pilocytic astrocytoma and clear cell ependymoma, as well as neuronal or glioneuronal tumors such as neurocytomas and dysembryoplastic neuroepithelial tumors (DNTs). The presence of Rosenthal fibers and eosinophilic granular bodies in a glial tumor suggests pilocytic astrocytoma, although they can be rarely found in diffuse gliomas as well. Other features that are helpful in this respect include biphasic growth pattern (pilocytic astrocytomas), the formation of true rosettes or perivascular pseudorosettes (ependymal tumors) and the formation of specific glioneuronal elements (DNT). Furthermore, immunohistochemical studies (e.g., dot-like staining for epithelial membrane antigen typically present in ependymal tumors) and occasionally electron microscopy (for ultrastructural demonstration of ependymal or neuronal differentiation) may be helpful in problematic cases. However, synaptophysin staining (for quite some time considered as sound evidence for neuronal rather than glial differentiation) has been described to occur in ‘bona fide’ oligodendrogliomas (and other gliomas) as well, indicating that the progenitor cells from which these tumors are derived are less strictly committed to glial lineage than previously thought [92, 123]. This may also explain that occasionally oligodendrogliomas are reported to display neurocytic or ganglioglioma-like maturation and that differentiation from other, more typical glioneuronal tumors can be challenging [91, 114, 115].
Unequivocal recognition of oligodendrogliomas is hindered by the lack of specific immunohistochemical markers for these tumors [89]. More recently described candidate markers in this respect are Olig2 (a murine bHLH transcription factor expressed in neural progenitors and oligodendroglia and considered to be essential for oligodendrocyte development) and the neuronal intermediate filament alpha internexin (INA). After initial optimism, Olig2 expression was not found helpful for distinguishing oligodendrogliomas from diffuse astrocytomas, nor from DNTs or pilocytic astrocytomas (although lack of Olig2 staining may aid in the diagnosis of neurocytoma or clear cell ependymoma rather than oligodendroglioma) [56, 72, 86, 93, 113]. Similarly, INA expression did not appear to provide the sensitivity and specificity needed for robust recognition of oligodendrogliomas [14, 26, 27]. Very recently, expression of phosphorylated cyclic-AMP responsive element binding protein (p-CREB, a transcription factor involved in gliomagenesis) was described to be present in astrocytomas but largely absent in prototypic oligodendrogliomas [8], but the sensitivity for identifying 1p19q codeleted oligodendrogliomas was only 70 % and the value of this tool for discrimination of diffuse gliomas awaits further elucidation.
While multiple studies show that distinctive histologic features in diffuse gliomas are associated with clinical outcome and with specific molecular alterations, the lack of robust histopathological criteria causes substantial diagnostic interobserver variability, even among experienced neuropathologists [36, 65]. This situation results in undesirable clinical discrepancies [117]. More precise classification of gliomas is urgently needed for adequate assessment of prognosis and appropriate planning of treatment. While improved definitions of morphological features may be helpful in this respect [64], a more promising approach is to integrate molecular data in order to create a more objective and reproducible glioma classification.
Molecular mechanisms and markers
Like other neoplasms, diffuse gliomas develop as a result of genetic and molecular alterations that further accumulate with tumor progression. These aberrations allow the tumor to acquire assets for sustained survival and growth and to escape normal growth restraining influences [39, 40]. Some of these molecular abnormalities carry important diagnostic, prognostic and/or predictive information, giving support for a major molecular component in future tumor characterization. The most promising markers for oligodendroglial tumors are briefly discussed below.
Complete 1p/19q codeletion
In 1994, using restriction fragment length polymorphism analysis, Reifenberger et al. [95] reported for the first time that many oligodendroglial tumors show loss of heterozygosity (LOH) for chromosome arms 1p and 19q. Further studies have shown that 1p/19q loss in fact results from a non-balanced translocation t (1:19) (q10:p10) with subsequent loss of one of the derivative chromosomes [38, 48]. This explains why it is the loss of the entire arms of 1p and 19q that is related to clinical outcome, rather than smaller terminal or interstitial deletions which occur by a completely different mechanism and are occasionally encountered in astrocytic tumors, such as glioblastoma [47]. Recently, by using genome-wide, 50 base pair single-end sequencing, 1p/19q codeletion was demonstrated to be the only copy number aberration that was stable across spatial regions of low-grade diffuse gliomas and their recurrences [122]. Virtually all 1p/19q codeleted tumors have a pro-neural expression profile, supporting the hypothesis that these gliomas originate from a bi-potential progenitor cell able to give rise to neurons and oligodendrocytes [25].
The high clinical relevance of 1p/19q loss became clear when this marker appeared to be associated with sensitivity to chemotherapy and improved outcome. Cairncross et al. [19] were the first to point out that recurrent anaplastic oligodendrogliomas with 1p/19q codeletion were far more responsive to PCV (procarbazine–ccnu–vincristine) chemotherapy, with virtually all tumors responding. In large prospective randomized studies on diffuse glioma, 1p/19q codeletion was associated with improved overall survival but also with increased benefit of adjuvant PCV chemotherapy given after radiotherapy [18, 118, 127].
Application of ‘strict’ (as opposed to ‘relaxed’) histopathological criteria for recognition of oligodendrogliomas results in a higher correlation with 1p/19q codeleted tumors [2, 51]. In a large series of ‘classic’ glioblastomas, 2–8 % of the primary/de novo and 0–13 % of secondary tumors were reported to show 1p/19q codeletion [83]. In some studies, GBM-Os showed a similar low frequency of 1p/19q codeletion [44], while others reported an increased frequency of codeletions in GBM-Os compared to conventional glioblastoma [4].
IHD1/IDH2 mutations
Following the initial discovery of isocitrate dehydrogenase (IDH) gene mutations in a small subset of especially younger glioblastoma patients with prolonged survival, a high percentage (70–80 %) of IDH1 and IDH2 mutations were identified in diffuse grade II and III gliomas, regardless of astrocytic or oligodendroglial cell type. Nearly all mutations involve codon 132 of IDH1 or the homologous codon 172 of IDH2, with c.395G>A (p.R132H) representing >90 % of all IDH1 mutations [87, 135]. The IDH1 R132H mutant protein can be detected reliably using immunohistochemistry [21]. This will, however, miss approximately 10 % of IDH mutations. The IDH mutation rate in 1p/19q codeleted tumors approaches 100 % [96, 124]. Further studies have shown that IDH mutation is an early event in gliomagenesis (‘driver mutation’), and likely precedes the development of the 1p/19q codeletion, the latter possibly specifically driving a diffuse glioma towards the classic oligodendroglioma morphologic phenotype, whereas superimposed TP53 and ATRX mutations drive it towards the astrocytoma phenotype instead [3, 124, 125].
Through an altered substrate specificity, IDH mutations give rise to metabolic alterations, including an increase in production of 2-hydroxyglutarate (2-HG) which can inhibit histone demethylation and induces a glioma hypermethylation phenotype (glioma CpG island methylated phenotype or G-CIMP) [45, 75, 116]. In turn, this leads to a variety of downstream chromatin remodeling and transcriptional alterations. IDH status has major prognostic implications for patients with diffuse gliomas, and was suggested to be a better predictive factor for benefit to chemotherapy in the RTOG trial on adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors [20]. IDH mutant tumors without 1p/19q codeletion have a worse outcome compared to IDH mutant, 1p/19q codeleted grade III tumors, but better than grade II and III tumors without IDH mutations.
MGMT promoter methylation and G-CIMP
O6-alkylguanine-DNA-alkyltransferase (AGT), encoded by the O6-methylguanine-DNA-methyltransferase (MGMT) gene, is a key repair enzyme that removes alkyl and methyl adducts from DNA, making cells with functional enzyme less sensitive to alkylating and methylating chemotherapy than those incapable of repairing these adducts. Indeed, gliomas with MGMT promoter methylation were demonstrated to be more sensitive to the alkylating agent temozolomide (TMZ) [43]. The biomarker value of immunohistochemistry for the repair enzyme itself is less clear [50, 94]. Later on, methylation of very specific regions in the MGMT promoter was found to be highly correlated to MGMT expression [6, 77].
Several studies have shown a high rate of MGMT promoter methylation in grade II and III gliomas, which subsequently was demonstrated to be related to IDH mutational status and G-CIMP. These findings at least partly explain the favorable outcome of MGMT methylated tumors after not only chemotherapy or combined chemoradiotherapy, but also after radiotherapy only [120, 127]. Genome-wide methylation studies have shown that MGMT promoter methylation is usually part of the G-CIMP phenotype, but about 10 % of G-CIMP positive tumors lack IDH mutation [82]. Virtually all 1p/19q codeleted oligodendrogliomas show G-CIMP and MGMT promoter methylation. With the Illumina 450 HM beadchip, a specific epigenetic pattern for IDH mutated and 1p/19q codeleted tumors can be identified [6, 121, 132]. With combined analysis of IDH, MGMT and G-CIMP status, it has become clear that the prognostic role of MGMT status in grade III tumors is related to IDH mutated tumors, whereas MGMT methylation status is predictive for benefit to alkylating chemotherapy in the absence of IDH mutations in both grade III and IV gliomas (i.e., high-grade astrocytomas) [121, 128, 132]. In EORTC study 26951 on anaplastic oligodendroglial tumors the methylation of specific regions in the MGMT promoter was found to be the strongest predictive factor for benefit to PCV chemotherapy [121].
CIC and FUBP1 mutations
Several other mutations have been identified in 1p/19q codeleted tumors. These include inactivating mutations in the homolog of the Drosophila capicua (CIC) and far-upstream binding protein 1 (FUBP1) gene, considered to occur secondary to the unbalanced translocation and found to be present in ~50–70 % and 15–30 % of 1p/19q codeleted tumors, respectively. These genes are located on the 19q (CIC) and 1p (FUBP1) chromosomal arms, supporting their putative roles as tumor suppressor genes [10]. No CIC mutations were identified in tumors without 19q loss [103]. The higher rate of CIC mutations in pure oligodendrogliomas suggests CIC mutations may be related to the phenotypic characteristics of these tumors [10, 54]. CIC mutations occur almost invariably with IDH mutations, and they are exceedingly rare in other brain tumors. CIC is a downstream component of receptor kinase (RTK) pathways (RTK–RAS–RAF–MAPK) and blocks transcription through binding to a regulatory region. It is negatively regulated by RTK signaling which blocks the function of CIC through MAPK-mediated phosphorylation. This results in the degradation of CIC. FUBP1 mutations may result in MYC activation or ribosome biogenesis. The additional impact of these alterations on the outcome of patients with 1p/19q codeleted glioma is presently unclear.
TERT and ATRX
Two mutually exclusive mutations play a role in telomere maintenance in gliomas: telomerase reverse transcriptase (TERT) mutations in hot spot promoter regions C228T, C250T result in increased expression of telomerase. Paradoxically, these mutations are present in both 1p/19q codeleted oligodendrogliomas and in IDH wild type high-grade gliomas [5, 59]. Virtually all 1p/19q codeleted tumors carry TERT mutations. Gliomas with TERT mutations in the presence of 1p/19q codeletion (i.e., oligodendrogliomas), have a very favorable outcome where the same TERT mutations in the absence of this codeletion or IDH mutations herald a grim prognosis (comparable to glioblastoma) [70]. TERT mutations are mutually exclusive with ATRX (alpha thalassemia mental retardation syndrome X linked) mutations, which are instead seen in the majority of WHO grade II-III astrocytomas and secondary glioblastomas. Critical for normal telomere homeostasis, these mutations are associated with the alternative lengthening of telomeres (ALT) phenotype, IDH and TP53 mutations, but not with 1p/19q loss [58, 62, 96, 104, 131]. Most diffuse gliomas thus show either ATRX or TERT mutations. The mechanisms involved in escaping senescence in the remaining set of gliomas still await further elucidation.
EGFR and chromosome 7
EGFR amplifications are virtually mutually exclusive with 1p/19q codeletion and with IDH mutations [52]. This suggests that these tumors follow a different oncogenic route from the start. The presence of polysomy of chromosome 7 and amplification of the epidermal growth factor receptor (EGFR) gene is associated with TERT mutations and a prognosis similar to primary glioblastoma in general. The presence of EGFR amplification in histologically pure anaplastic oligodendrogliomas is indicative of glioblastoma [31]. The small cell variant of glioblastoma has morphological similarities to anaplastic oligodendroglioma but carries EGFR amplification in 70 % of cases [90].
Anaplastic oligodendrogliomas
Anaplastic oligodendroglial tumors usually have additional genetic aberrations, in particular 9p LOH and/or deletion of the CDKN2A gene (p16), PIK3CA mutations, and polysomies [10, 11, 13, 31]. Recent data of The Cancer Genome Atlas (TCGA) consortium on lower grade (WHO grade II and III) gliomas show that IDH mutations in these gliomas are virtually mutually exclusive with homozygous deletion of CDKN2A and with amplification of EGFR [1, 12]. As discussed above, EGFR amplification mainly occurs in 1p/19q intact, IDH wild type gliomas and indicates the diagnosis glioblastoma. Some studies suggested shortened recurrence free survival times or poorer prognosis for oligodendroglial tumors with polysomy of 1p and 19q, but the exact importance of this finding is not yet clear [108, 129].
Pediatric oligodendroglial tumors
Pediatric and some young adulthood oligodendrogliomas are different from oligodendrogliomas in adults in that they show neither IDH mutations nor combined 1p/19q codeletion and thus represent a different disease [21, 63, 102, 111]. The rare childhood examples with IDH mutations and 1p19q codeletions almost always occur in adolescents, suggesting that they indeed carry the adult type of oligodendroglioma. In a similar vein, some young adult patients with tumors resembling oligodendroglioma, but lacking IDH mutations and 1p/19q codeletion may harbor the pediatric type of oligodendroglioma. This is similar to many other pediatric neoplasms that resemble an adult counterpart, but differ genetically and biologically [34, 110]. Given that it is difficult to define entities by a lack of findings, an objective definition for pediatric oligodendroglioma will likely remain a challenge until these tumors are better characterized at the molecular level. Recently, disseminated oligodendroglioma-like leptomeningeal neoplasms (an entity that predominantly affects children) were reported to frequently harbor concurrent BRAF-KIAA1549 gene fusions and 1p deletion, generally without 19q deletion [101].
Intratumoral genetic heterogeneity
In a recent study sequencing the exomes of 23 low-grade gliomas and their recurrences, in 43 % of cases, at least half of the mutations in the initial tumor were undetected at recurrence, including mutations considered to be driver mutations such as TP53 and ATRX. These findings suggest that recurrent tumors are often seeded by cells derived from the initial tumor at a very early stage of their evolution [55]. Indeed, other groups reported substantial intratumoral genetic heterogeneity (ITGH) in diffuse gliomas [88, 109, 122]. Adequate understanding of the consequences of such ITGH for the diagnosis and treatment of these tumors needs further study. Moreover, particular (chemo) therapeutic strategies/compounds may elicit certain molecular aberrations in tumor cells. In the study of Johnson et al. [55], tumors from 6 of 10 patients treated with the temozolomide (TMZ) were hypermutated and harbored driver mutations in the RB (retinoblastoma) and Akt-mTOR (mammalian target of rapamycin) pathways, thereby bearing the signature of TMZ-induced mutagenesis.
Who’s next?
The distinct differences in outcome of the various molecularly defined subsets even within morphologically similar types and grades of diffuse gliomas summarized above is now ready to be translated into a refined, more objective glioma classification with associated clinical, prognostic, and predictive implications. In fact, schemes were already proposed for a classification that is based on assessment of molecular aberrations rather than purely on histopathology [126]. Based on the available data, ‘canonical oligodendroglioma’ in adult patients could be defined as a diffuse glioma with complete 1p/19q codeletion, most of which will also have an IDH and a TERT mutation (Fig. 4b). Indeed, these tumors have the best outcome in the group of diffuse gliomas and show increased sensitivity to adjuvant PCV chemotherapy. Of note, 1p/19q status is most likely not the ultimate identifier of sensitivity to adjuvant (PCV) chemotherapy, better candidate markers being IDH mutation, G-CIMP and MGMT promoter methylation.
The question then arises how to call tumors with clear discrepancy between morphology and molecular information. Diffuse gliomas with intact 1p/19q may have oligodendroglial phenotype, but have a less favorable outcome especially if no IDH mutation is present. Vice versa, in many larger series on glioma a limited number of tumors diagnosed as low grade or anaplastic astrocytoma (5–10 %) has combined 1p/19q loss, most of these are IDH mutated. Several studies have now demonstrated that the molecular classification corresponds better with outcome than histopathology.
Recently, a group of neuropathologists with ample expertise in histopathological and molecular diagnosis of CNS tumors reached consensus and formulated the International Society of Neuropathology-Haarlem consensus guidelines (ISN-Haarlem guidelines) on how molecular information could be incorporated into an updated version of the WHO classification. Salient recommendations of this group included that: (1) diagnostic entities should be defined as narrowly as possible to optimize interobserver reproducibility, clinicopathological predictions and therapeutic planning; (2) diagnoses should be ‘layered’ with histologic classification, WHO grade, and molecular information listed below an ‘integrated diagnosis’ (Table 1); (3) determinations should be made for each tumor entity as to whether molecular information is required, suggested or not needed for its definition; (4) some pediatric entities should be separated from their adult counterparts [74]. The layered diagnostic format allows to leave the ‘molecular layer’ empty and to state in the integrated diagnosis that molecular testing was not performed. However, with the present status of molecular knowledge it is clear that molecular information is required for optimal diagnosis of diffuse gliomas and that in this context for most cases an ‘empty molecular layer’ must be viewed as shortcoming.
For molecular subtyping of diffuse gliomas several markers may be exploited (Fig. 4c). Elaborating on the suggestions in the ISN-Haarlem guidelines, a recent study on adult diffuse low- and high-grade gliomas demonstrated that the integrated morphological and molecular diagnosis (incorporating information on ATRX, IDH and 1p/19q codeletion) had significantly greater prognostic power for overall and progression-free survival than the original, histopathological diagnosis. Acknowledging that ATRX mutations in IDH mutant diffuse gliomas almost never co-occur with 1p/19q codeletions and result in loss of immunohistochemical ATRX staining of tumor cell nuclei, an algorithm was proposed for a stepwise diagnostic approach that could reduce the number of molecular analyses needed for a final diagnosis: initial immunohistochemistry for ATRX and IDH1 R132H mutant protein, when necessary (e.g., in case of positive ATRX staining of tumor cell nuclei and negative staining for IDH1 R132H) followed by 1p/19q analysis and subsequently by IDH sequencing [96]. Alternatively, assessment of TERT and/or TP53 mutations may be exploited for establishing such a prognostic classification, canonical oligodendrogliomas typically being TERT mutated and TP53 wild type (Fig. 4c). Immunohistochemistry for p53 protein (strong staining of a large percentage of tumor cell nuclei) has been reported as a highly specific, but moderately sensitive surrogate marker for the presence of a TP53 mutation in gliomas [112].
Several studies have now revealed that molecular and epigenetic/methylation characteristics allow reclassification of oligoastrocytomas or morphologically ambiguous diffuse gliomas into either oligodendroglioma or astrocytoma. Based on these conclusions, some authors suggest that it is time to say farewell to oligoastrocytoma as an entity [104, 133]. Indeed, the diagnosis of mixed gliomas has suffered amongst the greatest levels of discordance, even among expert neuropathologists. Already in 1997 the finding that low-grade oligoastrocytomas generally have either 1p/19q codeletion or TP53 mutation made the authors question the presence of truly mixed gliomas [76]. GBM-O is in fact nothing more than the grade IV variant of oligoastrocytoma and all of the caveats discussed above pertain. Distinct molecular subsets can easily be carved out of the GBM-O category and placed into more clinically uniform subtypes, such as primary/IDH wild type glioblastomas, secondary/IDH mutated glioblastomas, and 1p/19q codeleted anaplastic oligodendrogliomas. With the adoption of an integrated morphological and molecular diagnosis from a clinical perspective, the need for a separate mixed diffuse glioma (incl. GBM-O) category will largely disappear [32, 41, 53, 83].
The suggestion that it is time to say farewell to oligoastrocytoma as a diagnosis immediately elicited case reports of tumors showing molecular features of composite oligodendroglioma and astrocytoma [46, 134]. Evidently, while molecular classification allows for more stringent definition of the vast majority of diffuse gliomas, there will always be single cases that do not readily fit into the scheme. Further investigations are required to sort out if such ‘outliers’ justify the creation of separate diagnostic categories [105].
Introducing a new, integrated morphological and molecular definition of subgroups of diffuse gliomas will in itself already lead to a change in WHO grade in some cases (see Figs. 5, 6 for examples). Also, evaluation of the exact criteria to be used for grading and the significance of such grading within molecular subgroups is warranted [33]. A recent study of 558 WHO grade II and III diffuse gliomas revealed that mitotic index was significantly associated with outcome in IDH wild type but not in IDH mutant tumors [84].
Example of how ‘molecular reclassification’ may affect tumor grade. This tumor, previously diagnosed as GBM-O, WHO grade IV featured mostly cells resembling astrocytoma (a, c) and included necrosis (a) and microvascular proliferation (b). Cells resembling oligodendroglioma were seen only focally (d). The expression of IDH1-R132H mutant protein suggested that this represents either a secondary glioblastoma or an anaplastic oligodendroglioma, whereas the PTEN deletion identified by FISH is more common in glioblastoma (f; centromere 10 probe in green and PTEN probe in orange). Given the combined deletions of chromosome 1p (g; 1p probe in orange and 1q probe in green) and 19q (19p probe in green and 19q probe in orange), however, this case would be reclassified as an anaplastic oligodendroglioma, WHO grade III based on the most current recommendations
Example where ‘molecular reclassification’ does not affect tumor grade. This previously diagnosed oligoastrocytoma, WHO grade II (a) with IDH1 R132H mutant protein (b) would now be reclassified as a diffuse astrocytoma, WHO grade II based on strong p53 expression (c correlating well albeit imperfectly with TP53 mutation) and loss of ATRX expression (d note positive expression in non-neoplastic nuclei serving as a positive internal control)
Other challenges that need to be tackled are how to put pediatric oligodendrogliomas (known to lack 1p/19q codeletions) in the taxonomy of oligodendroglial tumors and which platforms and cut-off levels are the most ideal to be used for demonstration of particular molecular aberrations. The different platforms for detection of the relevant markers have their own strengths and weaknesses [45, 98]. Importantly, for recognition of the clinically relevant 1p/19q codeletion, assays capable of demonstrating whole-arm losses are preferable, in order to minimize the risk of false positives from incomplete 1p and 19q losses (Fig. 7).
For unequivocal assessment of 1p/19q codeletion in oligodendroglial tumors, detection of whole-arm losses is key. In order to avoid detection of false-positive cases with partial 1p and/or 19q loss, ideally a test is used that allows for analysis of multiple loci along each chromosome arm. a Example of an oligodendroglioma of which formalin-fixed, paraffin-embedded tissue was analyzed for 1p/19q status by multiplex ligation-dependent probe amplification (MLPA). With this platform, probes for 15 loci on chromosome 1p and 8 loci on 19q are used to interrogate these chromosome arms for loss or gain [the rest of the probes (3 on 1q, 2 on 19p, 15 on other chromosomes) serve as copy number references]. The test is performed in duplicate (hence the two lines) and demonstrates a clear loss for all 1p and 19q probes in this case (see [49] for technical details). b Massively parallel sequencing (MPS) allows for much more detailed analysis of copy number aberrations in the (tumor) genome. This is an example of a low-grade oligodendroglioma of which four different regions were separately analyzed. Interestingly, while complete losses of chromosome arms 1p (indicated by the arrow) and 19q (arrowhead) were uniformly present in the different regions, other copy number aberrations were only detected in some regions (most strikingly in area I, see region marked by red box). As the deletions for 1p and 19q loss in the different regions are at a similar level, this heterogeneity for copy number aberrations of other chromosomes cannot simply be explained by a difference in percentage of tumor cells in the different areas and must be interpreted as intratumoral genetic heterogeneity. Image b is modified from [122]
In the more distant future, the therapeutic management of individual patients may be determined by their ‘cancer signature’ rather than by the traditional pathological diagnosis [22]. For instance, the presence of an IDH mutation may provide an opportunity to apply specific, IDH targeted treatment, no matter if the diagnosis was astrocytoma or (1p/19q codeleted) oligodendroglioma [15, 106]. Furthermore, now that diffuse gliomas are increasingly diagnosed based on presence or absence of specific molecular aberrations, one can imagine that some of these tumors may be diagnosed by non- or minimally invasive approaches. Several groups already reported that molecular subgroups of gliomas (e.g., IDH mutant gliomas) may be recognized as such by advanced magnetic resonance evaluation [28, 30, 57]. Alternatively, ‘liquid biopsies’ may increasingly be used to detect particular markers in blood of glioma patients [9, 66]. It remains to be seen though if such non-invasive methods will ultimately provide information that is detailed and robust enough to replace tissue-based morphological and molecular analysis. Moreover, since the benefits of near-total resection of diffuse gliomas have long been recognized, tissue may continue to be obtained in most cases even if a diagnosis may be made by other means.
In conclusion, after almost a century of a histopathologically based classification of CNS tumors, we are now experiencing the transition towards an integrated morphological and molecular definition of diffuse gliomas, with in some situations the clinical relevance of the molecular findings overriding the relevance of the histopathological diagnosis. This integrated diagnostic approach allows for much more robust recognition of biologically different subgroups within the spectrum of diffuse gliomas, thereby facilitating tailored treatment for individual patients. The official updating process of the 4th edition of the WHO classification of CNS tumors has just begun and ‘the jury is still out’ with regard to what exactly the definitions for subgroups of diffuse gliomas will look like.
It can be expected that in the updated WHO classification (publication of which is scheduled early in 2016) demonstration of (complete) 1p/19q codeletion will indeed be required for the diagnosis of ‘canonical oligodendroglioma’ and that the (poorly reproducible) diagnosis of mixed gliomas will largely disappear. Such a change will bring new challenges as well, e.g., regarding how criteria for grading within the molecular groups should be adapted, how pediatric oligodendrogliomas should be defined, which platforms and cut-off levels should ideally be used for molecular diagnostics, and how to stay connected with centers/countries where molecular testing is not available. Also, acknowledging that our knowledge on the molecular underpinnings of cancer will continue to rapidly expand, it may well be necessary to prepare for more frequent updates of tumor classifications than we have seen so far. A multidisciplinary team-effort is needed to do derive the right balance between state-of-the-art science and clinical practicality in this respect [74, 99].
References
Agnihotri S, Aldape KD, Zadeh G (2014) Isocitrate dehydrogenase status and molecular subclasses of glioma and glioblastoma. Neurosurg Focus 37:E13. doi:10.3171/2014.9.FOCUS14505
Aldape K, Burger PC, Perry A (2007) Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma. Arch Pathol Lab Med 131:242–251. doi:10.1043/1543-2165(2007)131[242:CAOQLA]2.0.CO;2
Appin CL, Brat DJ (2015) Molecular pathways in gliomagenesis and their relevance to neuropathologic diagnosis. Adv Anat Pathol 22:50–58. doi:10.1097/PAP.0000000000000048
Appin CL, Gao J, Chisolm C, Torian M, Alexis D, Vincentelli C, Schniederjan MJ, Hadjipanayis C, Olson JJ, Hunter S et al (2013) Glioblastoma with oligodendroglioma component (GBM-O): molecular genetic and clinical characteristics. Brain Pathol 23:454–461. doi:10.1111/bpa.12018
Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Collins VP, Kawahara N et al (2013) Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol 126:267–276. doi:10.1007/s00401-013-1141-6
Bady P, Sciuscio D, Diserens AC, Bloch J, van den Bent MJ, Marosi C, Dietrich PY, Weller M, Mariani L, Heppner FL et al (2012) MGMT methylation analysis of glioblastoma on the infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol 124:547–560. doi:10.1007/s00401-012-1016-2
Baily P, Bucy PC (1929) Oligodendrogliomas of the brain. J Pathol Bacteriol 32:735–751
Barresi V, Mondello S, Branca G, Rajan TS, Vitarelli E, Tuccari G (2014) p-CREB expression in human gliomas: potential use in the differential diagnosis between astrocytoma and oligodendroglioma. Hum Pathol. doi:10.1016/j.humpath.2014.10.011
Best MG, Sol N, Zijl S, Reijneveld JC, Wesseling P, Wurdinger T (2015) Liquid biopsies in patients with diffuse glioma. Acta Neuropathol. doi:10.1007/s00401-015-1399-y
Bettegowda C, Agrawal N, Jiao Y, Sausen M, Wood LD, Hruban RH, Rodriguez FJ, Cahill DP, McLendon R, Riggins G et al (2011) Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science 333:1453–1455. doi:10.1126/science.1210557
Bigner SH, Matthews MR, Rasheed BK, Wiltshire RN, Friedman HS, Friedman AH, Stenzel TT, Dawes DM, McLendon RE, Bigner DD (1999) Molecular genetic aspects of oligodendrogliomas including analysis by comparative genomic hybridization. Am J Pathol 155:375–386. doi:10.1016/S0002-9440(10)65134-6
Brat DJ, Verhaak, R.G.W., Aldape, K.D. et al. (2015) Comprehensive, integrative genomic analysis of diffuse lower grade gliomas. N Engl J Med (in press)
Broderick DK, Di C, Parrett TJ, Samuels YR, Cummins JM, McLendon RE, Fults DW, Velculescu VE, Bigner DD, Yan H (2004) Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res 64:5048–5050. doi:10.1158/0008-5472.CAN-04-1170
Buckley PG, Alcock L, Heffernan J, Woods J, Brett F, Stallings RL, Farrell MA (2011) Loss of chromosome 1p/19q in oligodendroglial tumors: refinement of chromosomal critical regions and evaluation of internexin immunostaining as a surrogate marker. J Neuropathol Exp Neurol 70:177–182. doi:10.1097/NEN.0b013e31820c765b
Bunse L, Schumacher T, Sahm F, Pusch S, Oezen I, Rauschenbach K, Gonzalez M, Solecki G, Osswald M, Capper D et al (2015) Proximity ligation assay evaluates IDH1R132H presentation in gliomas. J Clin Investig 125:1–14. doi:10.1172/JCI77780
Burger PC (2002) What is an oligodendroglioma? Brain Pathol 12:257–259
Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP (2005) Years of life lost (YLL) from cancer is an important measure of population burden—and should be considered when allocating research funds. Br J Cancer 92:241–245. doi:10.1038/sj.bjc.6602321
Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W et al (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol Off J Am Soc Clin Oncol 31:337–343. doi:10.1200/JCO.2012.43.2674
Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y et al (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
Cairncross JG, Wang M, Jenkins RB, Shaw EG, Giannini C, Brachman DG, Buckner JC, Fink KL, Souhami L, Laperriere NJ et al (2014) Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol Off J Am Soc Clin Oncol 32:783–790. doi:10.1200/JCO.2013.49.3726
Capper D, Reuss D, Schittenhelm J, Hartmann C, Bremer J, Sahm F, Harter PN, Jeibmann A, von Deimling A (2011) Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol 121:241–252. doi:10.1007/s00401-010-0770-2
Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C (2013) Emerging landscape of oncogenic signatures across human cancers. Nat Genet 45:1127–1133. doi:10.1038/ng.2762
Claes A, Idema AJ, Wesseling P (2007) Diffuse glioma growth: a guerilla war. Acta Neuropathol 114:443–458. doi:10.1007/s00401-007-0293-7
Donahue B, Scott CB, Nelson JS, Rotman M, Murray KJ, Nelson DF, Banker FL, Earle JD, Fischbach JA, Asbell SO et al (1997) Influence of an oligodendroglial component on the survival of patients with anaplastic astrocytomas: a report of Radiation Therapy Oncology Group 83-02. Int J Radiat Oncol Biol Phys 38:911–914
Ducray F, Idbaih A, de Reynies A, Bieche I, Thillet J, Mokhtari K, Lair S, Marie Y, Paris S, Vidaud M et al (2008) Anaplastic oligodendrogliomas with 1p19q codeletion have a proneural gene expression profile. Mol cancer 7:41. doi:10.1186/1476-4598-7-41
Ducray F, Mokhtari K, Criniere E, Idbaih A, Marie Y, Dehais C, Paris S, Carpentier C, Dieme MJ, Adam C et al (2011) Diagnostic and prognostic value of alpha internexin expression in a series of 409 gliomas. Eur J Cancer 47:802–808. doi:10.1016/j.ejca.2010.11.031
Eigenbrod S, Roeber S, Thon N, Giese A, Krieger A, Grasbon-Frodl E, Egensperger R, Tonn JC, Kreth FW, Kretzschmar HA (2011) alpha-Internexin in the diagnosis of oligodendroglial tumors and association with 1p/19q status. J Neuropathol Exp Neurol 70:970–978. doi:10.1097/NEN.0b013e3182333ef5
Ellingson BM, Lai A, Harris RJ, Selfridge JM, Yong WH, Das K, Pope WB, Nghiemphu PL, Vinters HV, Liau LM et al (2013) Probabilistic radiographic atlas of glioblastoma phenotypes. Am J Neuroradiol 34:533–540. doi:10.3174/ajnr.A3253
Elmahdi A, Frary AJ, Scotton WJ, O’Donovan DG, Price SJ (2013) Glioblastomas with oligodendroglial component have the same clinical phenotype as classical glioblastomas. Br J Neurosurg 27:419–424. doi:10.3109/02688697.2013.767315
Esmaeili M, Hamans BC, Navis AC, van Horssen R, Bathen TF, Gribbestad IS, Leenders WP, Heerschap A (2014) IDH1 R132H mutation generates a distinct phospholipid metabolite profile in glioma. Cancer Res 74:4898–4907. doi:10.1158/0008-5472.CAN-14-0008
Fallon KB, Palmer CA, Roth KA, Nabors LB, Wang W, Carpenter M, Banerjee R, Forsyth P, Rich K, Perry A (2004) Prognostic value of 1p, 19q, 9p, 10q, and EGFR–FISH analyses in recurrent oligodendrogliomas. J Neuropathol Exp Neurol 63:314–322
Figarella-Branger D, Mokhtari K, Colin C, Uro-Coste E, Jouvet A, Dehais C, Carpentier C, Villa C, Maurage C, Eimer S et al (2014) Prognostic relevance of histomolecular classification of diffuse adult high-grade gliomas with necrosis. Brain Pathol. doi:10.1111/bpa.12227
Figarella-Branger D, Mokhtari K, Dehais C, Jouvet A, Uro-Coste E, Colin C, Carpentier C, Forest F, Maurage CA, Vignaud JM et al (2014) Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro oncol 16:1244–1254. doi:10.1093/neuonc/nou047
Gajjar A, Pfister SM, Taylor MD, Gilbertson RJ (2014) Molecular insights into pediatric brain tumors have the potential to transform therapy. Clin Cancer Res Off J Am Assoc Cancer Res 20:5630–5640. doi:10.1158/1078-0432.CCR-14-0833
Giannini C, Burger PC, Berkey BA, Cairncross JG, Jenkins RB, Mehta M, Curran WJ, Aldape K (2008) Anaplastic oligodendroglial tumors: refining the correlation among histopathology, 1p 19q deletion and clinical outcome in Intergroup Radiation Therapy Oncology Group Trial 9402. Brain Pathol 18:360–369. doi:10.1111/j.1750-3639.2008.00129.x
Giannini C, Scheithauer BW, Weaver AL, Burger PC, Kros JM, Mork S, Graeber MB, Bauserman S, Buckner JC, Burton J et al (2001) Oligodendrogliomas: reproducibility and prognostic value of histologic diagnosis and grading. J Neuropathol Exp Neurol 60:248–262
Goodenberger ML, Jenkins RB (2012) Genetics of adult glioma. Cancer Genet 205:613–621. doi:10.1016/j.cancergen.2012.10.009
Griffin CA, Burger P, Morsberger L, Yonescu R, Swierczynski S, Weingart JD, Murphy KM (2006) Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol 65:988–994. doi:10.1097/01.jnen.0000235122.98052.8f
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T et al (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120:707–718. doi:10.1007/s00401-010-0781-z
Heath JA, Ng J, Beshay V, Coleman L, Lo P, Amor DJ (2013) Anaplastic oligodendroglioma in an adolescent with Lynch syndrome. Pediatr Blood Cancer 60:E13–E15. doi:10.1002/pbc.24424
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Hegi ME, Janzer RC, Lambiv WL, Gorlia T, Kouwenhoven MC, Hartmann C, von Deimling A, Martinet D, Besuchet Schmutz N, Diserens AC et al (2012) Presence of an oligodendroglioma-like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta Neuropathol 123:841–852. doi:10.1007/s00401-011-0938-4
Horbinski C (2013) What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol 125:621–636. doi:10.1007/s00401-013-1106-9
Huse JT, Diamond EL, Wang L, Rosenblum MK (2015) Mixed glioma with molecular features of composite oligodendroglioma and astrocytoma: a true “oligoastrocytoma”? Acta Neuropathol 129:151–153. doi:10.1007/s00401-014-1359-y
Idbaih A, Marie Y, Pierron G, Brennetot C, Hoang-Xuan K, Kujas M, Mokhtari K, Sanson M, Lejeune J, Aurias A et al (2005) Two types of chromosome 1p losses with opposite significance in gliomas. Ann Neurol 58:483–487. doi:10.1002/ana.20607
Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, Flynn H, Passe S, Felten S, Brown PD et al (2006) A t (1:19) (q10:p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res 66:9852–9861. doi:10.1158/0008-5472.CAN-06-1796
Jeuken J, Cornelissen S, Boots-Sprenger S, Gijsen S, Wesseling P (2006) Multiplex ligation-dependent probe amplification: a diagnostic tool for simultaneous identification of different genetic markers in glial tumors. J Mol Diagn 8:433–443. doi:10.2353/jmoldx.2006.060012
Jeuken JW, Cornelissen SJ, Vriezen M, Dekkers MM, Errami A, Sijben A, Boots-Sprenger SH, Wesseling P (2007) MS-MLPA: an attractive alternative laboratory assay for robust, reliable, and semiquantitative detection of MGMT promoter hypermethylation in gliomas. Lab Investig J Tech Methods Pathol 87:1055–1065. doi:10.1038/labinvest.3700664
Jeuken JW, Sprenger SH, Boerman RH, von Deimling A, Teepen HL, van Overbeeke JJ, Wesseling P (2001) Subtyping of oligo-astrocytic tumours by comparative genomic hybridization. J Pathol 194:81–87. doi:10.1002/path.837
Jeuken JW, Sprenger SH, Wesseling P, Macville MV, von Deimling A, Teepen HL, van Overbeeke JJ, Boerman RH (1999) Identification of subgroups of high-grade oligodendroglial tumors by comparative genomic hybridization. J Neuropathol Exp Neurol 58:606–612
Jiang H, Ren X, Cui X, Wang J, Jia W, Zhou Z, Lin S (2013) 1p/19q codeletion and IDH1/2 mutation identified a subtype of anaplastic oligoastrocytomas with prognosis as favorable as anaplastic oligodendrogliomas. Neuro Oncol 15:775–782. doi:10.1093/neuonc/not027
Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, Rodriguez FJ, Rosemberg S, Oba-Shinjo SM, Nagahashi Marie SK et al (2012) Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 3:709–722
Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K et al (2014) Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343:189–193. doi:10.1126/science.1239947
Joseph NM, Phillips J, Dahiya S, Felicella M, Tihan T, Brat DJ, Perry A (2013) Diagnostic implications of IDH1-R132H and OLIG2 expression patterns in rare and challenging glioblastoma variants. Mod Pathol Off J US Can Acad Pathol. doi:10.1038/modpathol.2012.173
Kalinina J, Carroll A, Wang L, Yu Q, Mancheno DE, Wu S, Liu F, Ahn J, He M, Mao H et al (2012) Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. J Mol Med (Berl) 90:1161–1171. doi:10.1007/s00109-012-0888-x
Kannan K, Inagaki A, Silber J, Gorovets D, Zhang J, Kastenhuber ER, Heguy A, Petrini JH, Chan TA, Huse JT (2012) Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget 3:1194–1203
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC et al (2013) TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA 110:6021–6026. doi:10.1073/pnas.1303607110
Kleihues P, Cavenee WK (2000) Pathology and genetics of tumours of the nervous system: World Health Organization classification of tumours. IARC Press, Lyon
Koeller KK, Rushing EJ (2005) From the archives of the AFIP: oligodendroglioma and its variants: radiologic-pathologic correlation. Radiogr Rev Publ Radiol Soc N Am 25:1669–1688. doi:10.1148/rg.256055137
Koelsche C, Sahm F, Capper D, Reuss D, Sturm D, Jones DT, Kool M, Northcott PA, Wiestler B, Bohmer K et al (2013) Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol 126:907–915. doi:10.1007/s00401-013-1195-5
Kreiger PA, Okada Y, Simon S, Rorke LB, Louis DN, Golden JA (2005) Losses of chromosomes 1p and 19q are rare in pediatric oligodendrogliomas. Acta Neuropathol 109:387–392. doi:10.1007/s00401-004-0976-2
Kros JM (2011) Grading of gliomas: the road from eminence to evidence. J Neuropathol Exp Neurol 70:101–109. doi:10.1097/NEN.0b013e31820681aa
Kros JM, Gorlia T, Kouwenhoven MC, Zheng PP, Collins VP, Figarella-Branger D, Giangaspero F, Giannini C, Mokhtari K, Mork SJ et al (2007) Panel review of anaplastic oligodendroglioma from European Organization For Research and Treatment of Cancer Trial 26951: assessment of consensus in diagnosis, influence of 1p/19q loss, and correlations with outcome. J Neuropathol Exp Neurol 66:545–551. doi:10.1097/01.jnen.0000263869.84188.72
Kros JM, Mustafa DM, Dekker LJ, Sillevis Smitt PA, Luider TM, Zheng PP (2014) Circulating glioma biomarkers. Neuro Oncol. doi:10.1093/neuonc/nou207
Kros JM, Troost D, van Eden CG, van der Werf AJ, Uylings HB (1988) Oligodendroglioma. A comparison of two grading systems. Cancer 61:2251–2259
Kros JM, van den Brink WA, van Loon-van Luyt JJ, Stefanko SZ (1997) Signet-ring cell oligodendroglioma—report of two cases and discussion of the differential diagnosis. Acta Neuropathol 93:638–643
Kros JM, Van Eden CG, Stefanko SZ, Waayer-Van Batenburg M, van der Kwast TH (1990) Prognostic implications of glial fibrillary acidic protein containing cell types in oligodendrogliomas. Cancer 66:1204–1212
Labussiere M, Di Stefano AL, Gleize V, Boisselier B, Giry M, Mangesius S, Bruno A, Paterra R, Marie Y, Rahimian A et al (2014) TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations. Br J Cancer 111:2024–2032. doi:10.1038/bjc.2014.538
Li G, Zhang Z, Zhang J, Jin T, Liang H, Gong L, Cui G, Yang H, He S, Zhang Y et al (2014) Occipital anaplastic oligodendroglioma with multiple organ metastases after a short clinical course: a case report and literature review. Diagn Pathol 9:17. doi:10.1186/1746-1596-9-17
Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH (2004) The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol 63:499–509
Louis DN, Ohgaki H, Wiestler O, Cavenee WK (2007) WHO classification of tumours of the central nervous system. IARC Press, Lyon
Louis DN, Perry A, Burger P, Ellison DW, Reifenberger G, von Deimling A, Aldape K, Brat D, Collins VP, Eberhart C et al (2014) International society of neuropathology-haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol 24:429–435. doi:10.1111/bpa.12171
Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A et al (2012) IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483:474–478. doi:10.1038/nature10860
Maintz D, Fiedler K, Koopmann J, Rollbrocker B, Nechev S, Lenartz D, Stangl AP, Louis DN, Schramm J, Wiestler OD et al (1997) Molecular genetic evidence for subtypes of oligoastrocytomas. J Neuropathol Exp Neurol 56:1098–1104
Malley DS, Hamoudi RA, Kocialkowski S, Pearson DM, Collins VP, Ichimura K (2011) A distinct region of the MGMT CpG island critical for transcriptional regulation is preferentially methylated in glioblastoma cells and xenografts. Acta Neuropathol 121:651–661. doi:10.1007/s00401-011-0803-5
Mazza E, Belli C, Terreni M, Doglioni C, Losio C, Cantore M, Mambrini A, Reni M (2013) Breast metastases from oligodendroglioma: an unusual extraneural spread in two young women and a review of the literature. Crit Rev Oncol Hematol 88:564–572. doi:10.1016/j.critrevonc.2013.07.010
Michotte A, Chaskis C, Sadones J, Veld PI, Neyns B (2009) Primary leptomeningeal anaplastic oligodendroglioma with a 1p36-19q13 deletion: report of a unique case successfully treated with Temozolomide. J Neurol Sci 287:267–270. doi:10.1016/j.jns.2009.08.047
Miller CR, Dunham CP, Scheithauer BW, Perry A (2006) Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J Clin Oncol Off J Am Soc Clin Oncol 24:5419–5426. doi:10.1200/JCO.2006.08.1497
Miller CR, Perry A (2007) Glioblastoma. Arch Pathol Lab Med 131:397–406. doi:10.1043/1543-2165(2007)131[397:G]2.0.CO;2
Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522. doi:10.1016/j.ccr.2010.03.017
Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res Off J Am Assoc Cancer Res 19:764–772. doi:10.1158/1078-0432.CCR-12-3002
Olar A, Wani KM, Alfaro-Munoz KD, Heathcock LE, van Thuijl HF, Gilbert MR, Armstrong TS, Sulman EP, Cahill DP, Vera-Bolanos E et al (2015) IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol. doi:10.1007/s00401-015-1398-z
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol 16(Suppl 4):1–63. doi:10.1093/neuonc/nou223
Otero JJ, Rowitch D, Vandenberg S (2011) OLIG2 is differentially expressed in pediatric astrocytic and in ependymal neoplasms. J Neurooncol 104:423–438. doi:10.1007/s11060-010-0509-x
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. doi:10.1126/science.1164382
Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL et al (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344:1396–1401. doi:10.1126/science.1254257
Perry A (2001) Oligodendroglial neoplasms: current concepts, misconceptions, and folklore. Adv Anat Pathol 8:183–199
Perry A, Aldape KD, George DH, Burger PC (2004) Small cell astrocytoma: an aggressive variant that is clinicopathologically and genetically distinct from anaplastic oligodendroglioma. Cancer 101:2318–2326. doi:10.1002/cncr.20625
Perry A, Burton SS, Fuller GN, Robinson CA, Palmer CA, Resch L, Bigio EH, Gujrati M, Rosenblum MK (2010) Oligodendroglial neoplasms with ganglioglioma-like maturation: a diagnostic pitfall. Acta Neuropathol 120:237–252. doi:10.1007/s00401-010-0695-9
Perry A, Scheithauer BW, Macaulay RJ, Raffel C, Roth KA, Kros JM (2002) Oligodendrogliomas with neurocytic differentiation. A report of four cases with diagnostic and histogenetic implications. J Neuropathol Exp Neurol 61:947–955
Preusser M, Budka H, Rossler K, Hainfellner JA (2007) OLIG2 is a useful immunohistochemical marker in differential diagnosis of clear cell primary CNS neoplasms. Histopathology 50:365–370. doi:10.1111/j.1365-2559.2007.02614.x
Preusser M, Charles Janzer R, Felsberg J, Reifenberger G, Hamou MF, Diserens AC, Stupp R, Gorlia T, Marosi C, Heinzl H et al (2008) Anti-O6-methylguanine-methyltransferase (MGMT) immunohistochemistry in glioblastoma multiforme: observer variability and lack of association with patient survival impede its use as clinical biomarker. Brain Pathol 18:520–532. doi:10.1111/j.1750-3639.2008.00153.x
Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP (1994) Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol 145:1175–1190
Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, Schweizer L, Korshunov A, Jones DT, Hovestadt V et al (2015) ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol 129:133–146. doi:10.1007/s00401-014-1370-3
Reynolds RM, Boswell E, Hulette CM, Cummings TJ, Haglund MM, Boswell E, Hulette CM, Cumm Ings TJ, Haglund MM (2011) Sudden death from diffuse leptomeningeal oligodendrogliomatosis. J Neurosurg Spine 15:625–629. doi:10.3171/2011.7.SPINE10728
Riemenschneider MJ, Jeuken JW, Wesseling P, Reifenberger G (2010) Molecular diagnostics of gliomas: state of the art. Acta Neuropathol 120:567–584. doi:10.1007/s00401-010-0736-4
Riemenschneider MJ, Louis DN, Weller M, Hau P (2013) Refined brain tumor diagnostics and stratified therapies: the requirement for a multidisciplinary approach. Acta Neuropathol 126:21–37. doi:10.1007/s00401-013-1127-4
Rodriguez FJ, Perry A, Rosenblum MK, Krawitz S, Cohen KJ, Lin D, Mosier S, Lin MT, Eberhart CG, Burger PC (2012) Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity. Acta Neuropathol 124:627–641. doi:10.1007/s00401-012-1037-x
Rodriguez FJ, Schniederjan MJ, Nicolaides T, Tihan T, Burger PC, Perry A (2015) High rate of concurrent BRAF-KIAA1549 gene fusion and 1p deletion in disseminated oligodendroglioma-like leptomeningeal neoplasms (DOLN). Acta Neuropathol. doi:10.1007/s00401-015-1400-9
Rodriguez FJ, Tihan T, Lin D, McDonald W, Nigro J, Feuerstein B, Jackson S, Cohen K, Burger PC (2014) Clinicopathologic features of pediatric oligodendrogliomas: a series of 50 patients. Am J Surg Pathol 38:1058–1070. doi:10.1097/PAS.0000000000000221
Sahm F, Koelsche C, Meyer J, Pusch S, Lindenberg K, Mueller W, Herold-Mende C, von Deimling A, Hartmann C (2012) CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas. Acta Neuropathol 123:853–860. doi:10.1007/s00401-012-0993-5
Sahm F, Reuss D, Koelsche C, Capper D, Schittenhelm J, Heim S, Jones DT, Pfister SM, Herold-Mende C, Wick W et al (2014) Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol 128:551–559. doi:10.1007/s00401-014-1326-7
Sahm F, von Deimling A (2015) Farewell to oligoastrocytoma: response to letters. Acta Neuropathol 129:155. doi:10.1007/s00401-014-1366-z
Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M et al (2014) A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 512:324–327. doi:10.1038/nature13387
Smith SF, Simpson JM, Brewer JA, Sekhon LH, Biggs MT, Cook RJ, Little NS (2006) The presence of necrosis and/or microvascular proliferation does not influence survival of patients with anaplastic oligodendroglial tumours: review of 98 patients. J Neurooncol 80:75–82. doi:10.1007/s11060-006-9158-5
Snuderl M, Eichler AF, Ligon KL, Vu QU, Silver M, Betensky RA, Ligon AH, Wen PY, Louis DN, Iafrate AJ (2009) Polysomy for chromosomes 1 and 19 predicts earlier recurrence in anaplastic oligodendrogliomas with concurrent 1p/19q loss. Clin Cancer Res Off J Am Assoc Cancer Res 15:6430–6437. doi:10.1158/1078-0432.CCR-09-0867
Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavare S (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA 110:4009–4014. doi:10.1073/pnas.1219747110
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tonjes M, Sill M, Bender S et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437. doi:10.1016/j.ccr.2012.08.024
Suri V, Jha P, Agarwal S, Pathak P, Sharma MC, Sharma V, Shukla S, Somasundaram K, Mahapatra AK, Kale SS et al (2011) Molecular profile of oligodendrogliomas in young patients. Neuro oncol 13:1099–1106. doi:10.1093/neuonc/nor146
Takami H, Yoshida A, Fukushima S, Arita H, Matsushita Y, Nakamura T, Ohno M, Miyakita Y, Shibui S, Narita Y et al (2014) Revisiting TP53 mutations and immunohistochemistry-A comparative study in 157 diffuse gliomas. Brain Pathol. doi:10.1111/bpa.12173
Takei H, Yogeswaren ST, Wong KK, Mehta V, Chintagumpala M, Dauser RC, Lau CC, Adesina AM (2008) Expression of oligodendroglial differentiation markers in pilocytic astrocytomas identifies two clinical subsets and shows a significant correlation with proliferation index and progression free survival. J Neurooncol 86:183–190. doi:10.1007/s11060-007-9455-7
Takeuchi H, Kubota T, Kitai R, Matsuda K, Hashimoto N, Sato K (2009) Chromosome 1p and 19q deletions in malignant glioneuronal tumors with oligodendroglioma-like component. J Neurooncol 91:33–38. doi:10.1007/s11060-008-9690-6
Tanaka Y, Nobusawa S, Yagi S, Ikota H, Yokoo H, Nakazato Y (2012) Anaplastic oligodendroglioma with ganglioglioma-like maturation. Brain Tumor Pathol 29:221–228. doi:10.1007/s10014-011-0078-5
Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS et al (2012) IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483:479–483. doi:10.1038/nature10866
van den Bent MJ (2010) Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol 120:297–304. doi:10.1007/s00401-010-0725-7
van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, Bernsen HJ, Frenay M, Tijssen CC, Grisold W et al (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol Off J Am Soc Clin Oncol 31:344–350. doi:10.1200/JCO.2012.43.2229
van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L et al (2006) Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol Off J Am Soc Clin Oncol 24:2715–2722. doi:10.1200/JCO.2005.04.6078
van den Bent MJ, Dubbink HJ, Sanson M, van der Lee-Haarloo CR, Hegi M, Jeuken JW, Ibdaih A, Brandes AA, Taphoorn MJ, Frenay M et al (2009) MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. J Clin Oncol Off J Am Soc Clin Oncol 27:5881–5886. doi:10.1200/JCO.2009.24.1034
van den Bent MJ, Erdem-Eraslan L, Idbaih A, de Rooi J, Eilers PH, Spliet WG, den Dunnen WF, Tijssen C, Wesseling P, Sillevis Smitt PA et al (2013) MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res Off J Am Assoc Cancer Res 19:5513–5522. doi:10.1158/1078-0432.CCR-13-1157
van Thuijl HF, Scheinin I, Sie D, Alentorn A, van Essen HF, Cordes M, Fleischeuer R, Gijtenbeek AM, Beute G, van den Brink WA et al (2014) Spatial and temporal evolution of distal 10q deletion, a prognostically unfavorable event in diffuse low-grade gliomas. Genome Biol 15:471. doi:10.1186/s13059-014-0471-6
Vyberg M, Ulhoi BP, Teglbjaerg PS (2007) Neuronal features of oligodendrogliomas—an ultrastructural and immunohistochemical study. Histopathology 50:887–896. doi:10.1111/j.1365-2559.2007.02686.x
Wang XW, Ciccarino P, Rossetto M, Boisselier B, Marie Y, Desestret V, Gleize V, Mokhtari K, Sanson M, Labussiere M (2014) IDH mutations: genotype-phenotype correlation and prognostic impact. BioMed Res Int 2014:540236. doi:10.1155/2014/540236
Watanabe T, Nobusawa S, Kleihues P, Ohgaki H (2009) IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 174:1149–1153. doi:10.2353/ajpath.2009.080958
Weller M, Wick W (2014) Neuro-oncology in 2013: improving outcome in newly diagnosed malignant glioma. Nat Rev Neurol 10:68–70. doi:10.1038/nrneurol.2013.268
Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel MC, Koeppen S, Ketter R, Meyermann R et al (2009) NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol Off J Am Soc Clin Oncol 27:5874–5880. doi:10.1200/JCO.2009.23.6497
Wick W, Meisner C, Hentschel B, Platten M, Schilling A, Wiestler B, Sabel MC, Koeppen S, Ketter R, Weiler M et al (2013) Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology 81:1515–1522. doi:10.1212/WNL.0b013e3182a95680
Wiens AL, Cheng L, Bertsch EC, Johnson KA, Zhang S, Hattab EM (2012) Polysomy of chromosomes 1 and/or 19 is common and associated with less favorable clinical outcome in oligodendrogliomas: fluorescent in situ hybridization analysis of 84 consecutive cases. J Neuropathol Exp Neurol 71:618–624. doi:10.1097/NEN.0b013e31825b5f7a
Wieshmann UC, Milinis K, Paniker J, Das K, Jenkinson MD, Brodbelt A, Crooks D, Keller SS (2015) The role of the corpus callosum in seizure spread: MRI lesion mapping in oligodendrogliomas. Epilepsy Res 109:126–133. doi:10.1016/j.eplepsyres.2014.10.023
Wiestler B, Capper D, Holland-Letz T, Korshunov A, von Deimling A, Pfister SM, Platten M, Weller M, Wick W (2013) ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol 126:443–451. doi:10.1007/s00401-013-1156-z
Wiestler B, Capper D, Hovestadt V, Sill M, Jones DT, Hartmann C, Felsberg J, Platten M, Feiden W, Keyvani K et al (2014) Assessing CpG island methylator phenotype, 1p/19q codeletion, and MGMT promoter methylation from epigenome-wide data in the biomarker cohort of the NOA-04 trial. Neuro Oncol 16:1630–1638. doi:10.1093/neuonc/nou138
Wiestler B, Capper D, Sill M, Jones DT, Hovestadt V, Sturm D, Koelsche C, Bertoni A, Schweizer L, Korshunov A et al (2014) Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol 128:561–571. doi:10.1007/s00401-014-1315-x
Wilcox P, Li CC, Lee M, Shivalingam B, Brennan J, Suter CM, Kaufman K, Lum T, Buckland ME (2015) Oligoastrocytomas: throwing the baby out with the bathwater? Acta Neuropathol 129:147–149. doi:10.1007/s00401-014-1353-4
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. doi:10.1056/NEJMoa0808710
Zong H, Verhaak RG, Canoll P (2012) The cellular origin for malignant glioma and prospects for clinical advancements. Expert Rev Mol Diagn 12:383–394. doi:10.1586/erm.12.30
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wesseling, P., van den Bent, M. & Perry, A. Oligodendroglioma: pathology, molecular mechanisms and markers. Acta Neuropathol 129, 809–827 (2015). https://doi.org/10.1007/s00401-015-1424-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1424-1