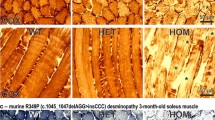
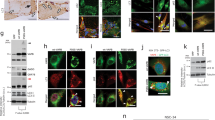
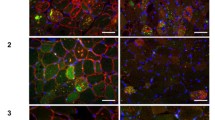
Abstract
Marinesco–Sjögren syndrome (MSS) features cerebellar ataxia, mental retardation, cataracts, and progressive vacuolar myopathy with peculiar myonuclear alterations. Most MSS patients carry homozygous or compound heterozygous SIL1 mutations. SIL1 is a nucleotide exchange factor for the endoplasmic reticulum resident chaperone BiP which controls a plethora of essential processes in the endoplasmic reticulum. In this study we made use of the spontaneous Sil1 mouse mutant woozy to explore pathomechanisms leading to Sil1 deficiency-related skeletal muscle pathology. We found severe, progressive myopathy characterized by alterations of the sarcoplasmic reticulum, accumulation of autophagic vacuoles, mitochondrial changes, and prominent myonuclear pathology including nuclear envelope and nuclear lamina alterations. These abnormalities were remarkably similar to the myopathy in human patients with MSS. In particular, the presence of perinuclear membranous structures which have been reported as an ultrastructural hallmark of MSS-related myopathy could be confirmed in woozy muscles. We found that these structures are derived from the nuclear envelope and nuclear lamina and associate with proliferations of the sarcoplasmic reticulum. In line with impaired function of BiP secondary to loss of its nucleotide exchange factor Sil1, we observed activation of the unfolded protein response and the endoplasmic-reticulum-associated protein degradation-pathway. Despite initiation of the autophagy–lysosomal system, autophagic clearance was found ineffective which is in agreement with the formation of autophagic vacuoles. This report identifies woozy muscle as a faithful phenocopy of the MSS myopathy. Moreover, we provide a link between two well-established disease mechanisms in skeletal muscle, dysfunction of chaperones and nuclear envelope pathology.






Similar content being viewed by others
References
Agbulut O, Destombes J, Thiesson D, Butler-Browne G (2000) Age-related appearance of tubular aggregates in the skeletal muscle of almost all male inbred mice. Histochem Cell Biol 114(6):477–481
Andreasson C, Rampelt H, Fiaux J, Druffel-Augustin S, Bukau B (2010) The endoplasmic reticulum Grp170 acts as a nucleotide exchange factor of Hsp70 via a mechanism similar to that of the cytosolic Hsp110. J Biol Chem 285(16):12445–12453. doi:10.1074/jbc.M109.096735
Anttonen AK, Mahjneh I, Hamalainen RH, Lagier-Tourenne C, Kopra O, Waris L, Anttonen M, Joensuu T, Kalimo H, Paetau A, Tranebjaerg L, Chaigne D, Koenig M, Eeg-Olofsson O, Udd B, Somer M, Somer H, Lehesjoki AE (2005) The gene disrupted in Marinesco–Sjogren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet 37(12):1309–1311. doi:10.1038/ng1677
Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20(1):31–42. doi:10.1038/cdd.2012.81
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171(4):603–614. doi:10.1083/jcb.200507002
Bjorkoy G, Lamark T, Johansen T (2006) p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy 2(2):138–139. (pii:2405)
Buchkovich NJ, Maguire TG, Alwine JC (2010) Role of the endoplasmic reticulum chaperone BiP, SUN domain proteins, and dynein in altering nuclear morphology during human cytomegalovirus infection. J Virol 84(14):7005–7017. doi:10.1128/JVI.00719-10
Dechat T, Korbei B, Vaughan OA, Vlcek S, Hutchison CJ, Foisner R (2000) Lamina-associated polypeptide 2alpha binds intranuclear A-type lamins. J Cell Sci 113(Pt 19):3473–3484
Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD (2008) Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 22(7):832–853. doi:10.1101/gad.1652708
Dubowitz V, Sewry CA, Oldfors A (2013) Muscle biopsy. A practical approach, Saunders
Dudek J, Benedix J, Cappel S, Greiner M, Jalal C, Muller L, Zimmermann R (2009) Functions and pathologies of BiP and its interaction partners. Cell Mol Life Sci 66(9):1556–1569. doi:10.1007/s00018-009-8745-y
Eskelinen EL, Schmidt CK, Neu S, Willenborg M, Fuertes G, Salvador N, Tanaka Y, Lullmann-Rauch R, Hartmann D, Heeren J, von Figura K, Knecht E, Saftig P (2004) Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol Biol Cell 15(7):3132–3145. doi:10.1091/mbc.E04-02-0103
Fatma N, Singh P, Chhunchha B, Kubo E, Shinohara T, Bhargavan B, Singh DP (2011) Deficiency of Prdx6 in lens epithelial cells induces ER stress response-mediated impaired homeostasis and apoptosis. Am J Physiol Cell Physiol 301(4):C954–C967. doi:10.1152/ajpcell.00061.2011
Fewell SW, Day BW, Brodsky JL (2001) Identification of an inhibitor of hsc70-mediated protein translocation and ATP hydrolysis. J Biol Chem 276(2):910–914. doi:10.1074/jbc.M008535200
Goto Y, Komiyama A, Tanabe Y, Katafuchi Y, Ohtaki E, Nonaka I (1990) Myopathy in Marinesco–Sjogren syndrome: an ultrastructural study. Acta Neuropathol 80(2):123–128
Haas IG, Wabl M (1983) Immunoglobulin heavy chain binding protein. Nature 306(5941):387–389
Harms MB, Sommerville RB, Allred P, Bell S, Ma D, Cooper P, Lopate G, Pestronk A, Weihl CC, Baloh RH (2012) Exome sequencing reveals DNAJB6 mutations in dominantly-inherited myopathy. Ann Neurol 71(3):407–416. doi:10.1002/ana.22683
Harting I, Blaschek A, Wolf NI, Seitz A, Haupt M, Goebel HH, Rating D, Sartor K, Ebinger F (2004) T2-hyperintense cerebellar cortex in Marinesco–Sjogren syndrome. Neurology 63(12):2448–2449. doi:63/12/2448
Herva R, von Wendt L, von Wendt G, Saukkonen AL, Leisti J, Dubowitz V (1987) A syndrome with juvenile cataract, cerebellar atrophy, mental retardation and myopathy. Neuropediatrics 18(3):164–169. doi:10.1055/s-2008-1052473
Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 25(2):193–205. doi:10.1016/j.molcel.2006.12.009
Izawa I, Nishizawa M, Ohtakara K, Ohtsuka K, Inada H, Inagaki M (2000) Identification of Mrj, a DnaJ/Hsp40 family protein, as a keratin 8/18 filament regulatory protein. J Biol Chem 275(44):34521–34527. doi:10.1074/jbc.M003492200
Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf DH, Sommer T (2002) Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat Cell Biol 4(2):134–139. doi:10.1038/ncb746
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18(4):571–580. doi:10.1038/cdd.2010.191
Kobayashi T, Ohta Y (2003) Enforced expression of oxygen-regulated protein, ORP150, induces vacuolar degeneration in mouse myocardium. Transgenic Res 12(1):13–22
Kobayashi T, Takita Y, Suzuki A, Katsu Y, Iguchi T, Ohta Y (2008) Vacuolar degeneration of skeletal muscle in transgenic mice overexpressing ORP150. J Vet Med Sci 70(1):115–118. doi:JST.JSTAGE/jvms/70.115
Kollmann K, Damme M, Markmann S, Morelle W, Schweizer M, Hermans-Borgmeyer I, Rochert AK, Pohl S, Lubke T, Michalski JC, Kakela R, Walkley SU, Braulke T (2012) Lysosomal dysfunction causes neurodegeneration in mucolipidosis II ‘knock-in’ mice. Brain 135(Pt 9):2661–2675. doi:10.1093/brain/aws209
Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131(6):1149–1163. doi:10.1016/j.cell.2007.10.035
Komiyama A, Nonaka I, Hirayama K (1989) Muscle pathology in Marinesco–Sjogren syndrome. J Neurol Sci 89(1):103–113
Krieger M, Roos A, Stendel C, Claeys KG, Sonmez FM, Baudis M, Bauer P, Bornemann A, de Goede C, Dufke A, Finkel RS, Goebel HH, Häussler M, Kingston H, Kirschner J, Medne L, Muschke P, Rivier F, Rudnik-Schöneborn S, Spengler S, Inzana F, Stanzial F, Benedicenti F, Synofzik M, Taratuto AL, Pirra L, Tay SK-H, Topaloglu H, Uyanik G, Wand D, Williams D, Zerres K, Weis J, Senderek J (2013) SIL1 mutations and clinical spectrum in patients with Marinesco–Sjögren syndrome. Brain Accepted for publication
Kudlow BA, Kennedy BK, Monnat RJ Jr (2007) Werner and Hutchinson–Gilford progeria syndromes: mechanistic basis of human progeroid diseases. Nat Rev Mol Cell Biol 8(5):394–404. doi:10.1038/nrm2161
Kuga T, Nozaki N, Matsushita K, Nomura F, Tomonaga T (2010) Phosphorylation statuses at different residues of lamin B2, B1, and A/C dynamically and independently change throughout the cell cycle. Exp Cell Res 316(14):2301–2312. doi:10.1016/j.yexcr.2010.05.017
Lee HS, Daniels BH, Salas E, Bollen AW, Debnath J, Margeta M (2012) Clinical utility of LC3 and p62 immunohistochemistry in diagnosis of drug-induced autophagic vacuolar myopathies: a case-control study. PLoS One 7(4):e36221. doi:10.1371/journal.pone.0036221
Li H, Chen Q, Liu F, Zhang X, Li W, Liu S, Zhao Y, Gong Y, Yan C (2013) Unfolded protein response and activated degradative pathways regulation in GNE myopathy. PLoS One 8(3):e58116. doi:10.1371/journal.pone.0058116
Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS (2008) The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ 15(9):1460–1471. doi:10.1038/cdd.2008.81
Liu GH, Qu J, Suzuki K, Nivet E, Li M, Montserrat N, Yi F, Xu X, Ruiz S, Zhang W, Wagner U, Kim A, Ren B, Li Y, Goebl A, Kim J, Soligalla RD, Dubova I, Thompson J, Yates J 3rd, Esteban CR, Sancho-Martinez I, Izpisua Belmonte JC (2012) Progressive degeneration of human neural stem cells caused by pathogenic LRRK2. Nature 491(7425):603–607. doi:10.1038/nature11557
Liu GL, Yu F, Dai DZ, Zhang GL, Zhang C, Dai Y (2012) Endoplasmic reticulum stress mediating downregulated StAR and 3-beta-HSD and low plasma testosterone caused by hypoxia is attenuated by CPU86017-RS and nifedipine. J Biomed Sci 19:4. doi:10.1186/1423-0127-19-4
Longatti A, Tooze SA (2012) Recycling endosomes contribute to autophagosome formation. Autophagy 8(11):1682–1683. doi:10.4161/auto.21486
Macario AJ, Cappello F, Zummo G, Conway de Macario E (2010) Chaperonopathies of senescence and the scrambling of interactions between the chaperoning and the immune systems. Ann N Y Acad Sci 1197:85–93. doi:10.1111/j.1749-6632.2010.05187.x
Macario AJ, Conway de Macario E (2007) Chaperonopathies and chaperonotherapy. FEBS Lett 581(19):3681–3688. doi:10.1016/j.febslet.2007.04.030
Macario AJ, Conway de Macario E (2007) Chaperonopathies by defect, excess, or mistake. Ann N Y Acad Sci 1113:178–191. doi:10.1196/annals.1391.009
Marinescu G, Draganesco S, Vasiliu D (1931) Nouvelle maladie familiale caractérisée par une cataracte congénitale et un arrêt du développement somato-neuro-psychique. Encéphale
Munro S, Pelham HR (1986) An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46(2):291–300. doi:0092-8674(86)90746-4
Namba T, Ishihara T, Tanaka K, Hoshino T, Mizushima T (2007) Transcriptional activation of ATF6 by endoplasmic reticulum stressors. Biochem Biophys Res Commun 355(2):543–548. doi:10.1016/j.bbrc.2007.02.004
Nijholt DA, de Graaf TR, van Haastert ES, Oliveira AO, Berkers CR, Zwart R, Ovaa H, Baas F, Hoozemans JJ, Scheper W (2011) Endoplasmic reticulum stress activates autophagy but not the proteasome in neuronal cells: implications for Alzheimer’s disease. Cell Death Differ 18(6):1071–1081. doi:10.1038/cdd.2010.176
Nishikawa S, Brodsky JL, Nakatsukasa K (2005) Roles of molecular chaperones in endoplasmic reticulum (ER) quality control and ER-associated degradation (ERAD). J Biochem 137(5):551–555. doi:10.1093/jb/mvi068
Oda Y, Okada T, Yoshida H, Kaufman RJ, Nagata K, Mori K (2006) Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J Cell Biol 172(3):383–393. doi:10.1083/jcb.200507057
Oh SH, Lim SC (2009) Endoplasmic reticulum stress-mediated autophagy/apoptosis induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal-regulated kinase activation in WI38 lung epithelial fibroblast cells. J Pharmacol Exp Ther 329(1):112–122. doi:10.1124/jpet.108.144113
Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS, Dauer W, Consiglio A, Raya A, Sulzer D, Cuervo AM (2013) Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci 16(4):394–406. doi:10.1038/nn.3350
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282(33):24131–24145. doi:10.1074/jbc.M702824200
Park YE, Hayashi YK, Bonne G, Arimura T, Noguchi S, Nonaka I, Nishino I (2009) Autophagic degradation of nuclear components in mammalian cells. Autophagy 5(6):795–804. doi:8901
Prell T, Lautenschlager J, Grosskreutz J (2013) Calcium-dependent protein folding in amyotrophic lateral sclerosis. Cell Calcium 54(2):132–143. doi:10.1016/j.ceca.2013.05.007
Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S (2002) AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol 22(2):626–634
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529. doi:10.1038/nrm2199
Salton M, Elkon R, Borodina T, Davydov A, Yaspo ML, Halperin E, Shiloh Y (2011) Matrin 3 binds and stabilizes mRNA. PLoS One 6(8):e23882. doi:10.1371/journal.pone.0023882
Sarparanta J, Jonson PH, Golzio C, Sandell S, Luque H, Screen M, McDonald K, Stajich JM, Mahjneh I, Vihola A, Raheem O, Penttila S, Lehtinen S, Huovinen S, Palmio J, Tasca G, Ricci E, Hackman P, Hauser M, Katsanis N, Udd B (2012) Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nat Genet 44(4):450–455. doi:10.1038/ng.1103 S451–452
Sasaki K, Suga K, Tsugawa S, Sakuma K, Tachi N, Chiba S, Imamura S (1996) Muscle pathology in Marinesco–Sjogren syndrome: a unique ultrastructural feature. Brain Dev 18(1):64–67. doi:0387760495000968
Schröder JM (1982) Pathologie der Muskulatur. Spezielle pathologische Anatomie, vol 15. Springer Verlag, Berlin
Schroder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789. doi:10.1146/annurev.biochem.73.011303.074134
Senderek J, Garvey SM, Krieger M, Guergueltcheva V, Urtizberea A, Roos A, Elbracht M, Stendel C, Tournev I, Mihailova V, Feit H, Tramonte J, Hedera P, Crooks K, Bergmann C, Rudnik-Schoneborn S, Zerres K, Lochmuller H, Seboun E, Weis J, Beckmann JS, Hauser MA, Jackson CE (2009) Autosomal-dominant distal myopathy associated with a recurrent missense mutation in the gene encoding the nuclear matrix protein, matrin 3. Am J Hum Genet 84(4):511–518
Senderek J, Krieger M, Stendel C, Bergmann C, Moser M, Breitbach-Faller N, Rudnik-Schoneborn S, Blaschek A, Wolf NI, Harting I, North K, Smith J, Muntoni F, Brockington M, Quijano-Roy S, Renault F, Herrmann R, Hendershot LM, Schroder JM, Lochmuller H, Topaloglu H, Voit T, Weis J, Ebinger F, Zerres K (2005) Mutations in SIL1 cause Marinesco–Sjogren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet 37(12):1312–1314
Sewry CA, Voit T, Dubowitz V (1988) Myopathy with unique ultrastructural feature in Marinesco–Sjogren syndrome. Ann Neurol 24(4):576–580. doi:10.1002/ana.410240416
Shen Y, Hendershot LM (2005) ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP’s interactions with unfolded substrates. Mol Biol Cell 16(1):40–50. doi:10.1091/mbc.E04-05-0434
Sjogren T (1950) Hereditary congenital spinocerebellar ataxia accompanied by congenital cataract and oligophrenia; a genetic and clinical investigation. Confin Neurol 10(5):293–308
Su TR, Tsai FJ, Lin JJ, Huang HH, Chiu CC, Su JH, Yang YT, Chen JY, Wong BS, Wu YJ (2012) Induction of apoptosis by 11-dehydrosinulariolide via mitochondrial dysregulation and ER stress pathways in human melanoma cells. Mar Drugs 10(8):1883–1898. doi:10.3390/md10081883
Superneau DW, Wertelecki W, Zellweger H, Bastian F (1987) Myopathy in Marinesco–Sjogren syndrome. Eur Neurol 26(1):8–16
Suzuki Y, Murakami N, Goto Y, Orimo S, Komiyama A, Kuroiwa Y, Nonaka I (1997) Apoptotic nuclear degeneration in Marinesco–Sjogren syndrome. Acta Neuropathol 94(5):410–415
Taylor MR, Slavov D, Gajewski A, Vlcek S, Ku L, Fain PR, Carniel E, Di Lenarda A, Sinagra G, Boucek MM, Cavanaugh J, Graw SL, Ruegg P, Feiger J, Zhu X, Ferguson DA, Bristow MR, Gotzmann J, Foisner R, Mestroni L (2005) Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum Mutat 26(6):566–574. doi:10.1002/humu.20250
Tirasophon W, Lee K, Callaghan B, Welihinda A, Kaufman RJ (2000) The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev 14(21):2725–2736
Tyson JR, Stirling CJ (2000) LHS1 and SIL1 provide a lumenal function that is essential for protein translocation into the endoplasmic reticulum. EMBO J 19(23):6440–6452. doi:10.1093/emboj/19.23.6440
Vattemi G, Engel WK, McFerrin J, Askanas V (2004) Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol 164(1):1–7. doi:10.1016/S0002-9440(10)63089-1
Villa A, Podini P, Nori A, Panzeri MC, Martini A, Meldolesi J, Volpe P (1993) The endoplasmic reticulum-sarcoplasmic reticulum connection. II. Postnatal differentiation of the sarcoplasmic reticulum in skeletal muscle fibers. Exp Cell Res 209(1):140–148. doi:S0014482783712942
Villa A, Podini P, Panzeri MC, Soling HD, Volpe P, Meldolesi J (1993) The endoplasmic-sarcoplasmic reticulum of smooth muscle: immunocytochemistry of vas deferens fibers reveals specialized subcompartments differently equipped for the control of Ca2+ homeostasis. J Cell Biol 121(5):1041–1051
Vlcek S, Dechat T, Foisner R (2001) Nuclear envelope and nuclear matrix: interactions and dynamics. Cell Mol Life Sci 58(12–13):1758–1765
Wang X, Fan H, Ying Z, Li B, Wang H, Wang G (2010) Degradation of TDP-43 and its pathogenic form by autophagy and the ubiquitin-proteasome system. Neurosci Lett 469(1):112–116. doi:10.1016/j.neulet.2009.11.055
Weis J, Dimpfel W, Schroder JM (1995) Nerve conduction changes and fine structural alterations of extra- and intrafusal muscle and nerve fibers in streptozotocin diabetic rats. Muscle Nerve 18(2):175–184
Weitzmann A, Volkmer J, Zimmermann R (2006) The nucleotide exchange factor activity of Grp170 may explain the non-lethal phenotype of loss of Sil1 function in man and mouse. FEBS Lett 580(22):5237–5240. doi:10.1016/j.febslet.2006.08.055
Wilkie GS, Schirmer EC (2006) Guilt by association: the nuclear envelope proteome and disease. Mol Cell Proteomics 5(10):1865–1875. doi:10.1074/mcp.R600003-MCP200
Worman HJ, Ostlund C, Wang Y (2010) Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol 2(2):a000760. doi:10.1101/cshperspect.a000760
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13(3):365–376. doi:10.1016/j.devcel.2007.07.018
Ye Y, Meyer HH, Rapoport TA (2001) The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414(6864):652–656. doi:10.1038/414652a
Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281(40):30299–30304. doi:10.1074/jbc.M607007200
Zhao L, Longo-Guess C, Harris BS, Lee JW, Ackerman SL (2005) Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet 37(9):974–979. doi:10.1038/ng1620
Zhao L, Rosales C, Seburn K, Ron D, Ackerman SL (2010) Alteration of the unfolded protein response modifies neurodegeneration in a mouse model of Marinesco–Sjogren syndrome. Hum Mol Genet 19(1):25–35. doi:10.1093/hmg/ddp464
Acknowledgments
We thank Hannelore Mader, Astrid Knischewski, Claudia Krude, Elke Beck, Hannelore Wiederhold, and Evelyne Pascual for expert technical assistance. We also thank Torsten Rollar for expert IT assistance. This work has been supported by a grant from START program of RWTH Aachen University (to A. R.; Grant No. 41/12), by the Else Kröner-Fresenius Stiftung (to A. R.; Grant No. A59/09), by the Ministerium für Innovation, Wissenschaft und Forschung des Landes Nordrhein-Westfalen, by a grant from the Maximilian-May-Stiftung (to J. S.) and grants by the German Research Foundation (DFG; WE 1406/13-1 and ZA 639/1-1) and the IZKF Aachen (to J. W.; Grant No. N5-3). Purchase of woozy animals was funded by the HOMFOR program.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2013_1224_MOESM3_ESM.ppt
Suppl. Fig. 1 Cryostat section histology of human and mouse muscle and macroscopic appearance of wz mouse hind limbs. (a) Vacuolar myopathy in the muscle biopsy of patient MSS33. Arrows: subsarcolemmal autophagic vacuoles, Gomori trichrome; scale bar = 23 μm. (b) Normal muscle fibers in 16 wk old wt mouse quadriceps muscle. PAS; scale bar = 26 μm. (c) Largely normal light microscopical appearance of wz littermate mouse quadriceps muscle at 16 weeks of age. Arrow: non-subsarcolemmal myonucleus. PAS; scale bar = 26 μm. (d) Tubular aggregates (arrow) in otherwise normal muscle fibers of an 84-week-old wt mouse. H&E; scale bar = 25 μm. (e) Vacuolar myopathy with highly variable muscle fiber calibers and nseveral non-subsarcolemmal myonuclei in wz littermate mouse. Arrows: rimmed vacuoles associated with myonuclear degeneration. H&E; scale bar = 20 μm. Inset: another rimmed vacuole with degenerated myonucleus. H&E; scale bar = 20 μm. (f) Tubular aggregates (red; arrow) in the 84-week-old wt mouse. Gomori trichrome; scale bar = 20 μm. (g) Condensed myonuclei (white arrow) in a damaged muscle fiber of the 84-week-old wz mouse. Black arrow: Subsarcolemmal rimmed vacuole, possibly associated with myonucleus. Tubular aggregates were absent from this muscle. Gomori trichrome; scale bar = 15 μm. (h) Normal muscle fibers of a 100-week-old wt mouse. H&E; scale bar = 25 μm. (i) Prominent vacuolar myopathy characterized by fiber atrophy and splitting (black arrow), moderate endomysial fibrosis and numerous non-subsarcolemmal muscle fiber nuclei as well as autophagic vacuoles derived from degenerating myonuclei (white arrows) in a 100-week-old wz littermate. H&E; scale bar in (i) = 20 μm and in inset = 15 μm. (j and l) Normal macroscopic appearance of hind limb muscle (black arrows: quadriceps muscle) in an 84- and a 100-week-old wt mouse. White arrows: normal fat tissue. (k and m) Reduced muscle bulk and vanished fat tissue in wz littermates. (PPT 449 kb)
401_2013_1224_MOESM4_ESM.ppt
Suppl. Fig. 2 Further histopathological findings in semithin sections of glutaraldehyde-fixed, epoxy resin-embedded muscle biopsy specimens of MSS patients (a and b) and wz mice (c-h). (a) Autophagic vacuole in the perinuclear sarcoplasm of a muscle fiber in the biopsy of MSS patient 3 (black arrow). White arrows: myofibrillar disintegration associated with prominent Z-Band streaming. Scale bar = 18 μm. (b) Large subsarcolemmal autophagic vacuole in a muscle fiber in the biopsy of MSS patient 33 (black arrow). Scale bar = 20 μm. (c) Muscle fibers without structural abnormalities and with normal nuclei in a 16-week-old control mouse. Longitudinal section. Scale bar = 20 μm. (d and e) Autophagic vacuoles in the perinuclear sarcoplasm of a muscle fiber of a 16-week-old wz mouse (black arrows). Scale bar in e = 15 μm, in f = 10 μm. (f and g) Increased number of non-subsarcolemmal nuclei in muscle fibers of 26-week-old wz mice (white arrows). Black arrows: autophagic vacuoles. Scale bar in g = 25 μm, in h = 20 μm. (Inset in h) Abnormal non-subsarcolemmal myonucleus (white arrow) with condensed chromatin and perinuclear membranous structure. Scale bar = 13 μm. (h) Large granular material in myonuclei of an 84-week-old wz mouse. Gray arrow: accumulation of moderately osmiophilic material (glycogen and mitochondria) in a large muscle fiber. Black arrow and inset: prominent subsarcolemmal myonuclei with remodeled chromatin. Scale bars in main figure and in inset = 20 μm. (PPT 7562 kb)
401_2013_1224_MOESM5_ESM.ppt
Suppl. Fig. 3 Ultrastructural alterations in wz mouse muscle at age 16 weeks analyzed by transmission electron microscopy. (a) Focal widening of the SR (black arrows) associated with accumulated autophagic material (white arrow). Scale bar = 2.5 μm. (b and c) Subsarcolemmal enlargements of SR. Scale bars = 0.8 μm (b) and 1.2 μm (c). (d) Mitochondria engulfed by multiple osmiophilic membranes indicating early mitophagia (black arrow). Scale bar = 0.7 μm. μm. (e) Membranous inclusion in muscle fiber mitochondrion (black arrow). White arrow: widened SR tubules. Scale bar = 0.5 μm. (f) Large perinuclear autophagic vacuole containing myelin-like osmiophilic material (black arrows) engulfing tubulovesicular structures, presumably SR. Scale bar = 1.3 μm. (g) Membranous autophagic material (black arrow) in the perinuclear sarcoplasm. Scale bar = 3.5 μm. (h) Larger accumulation of subsarcolemmal membranous autophagic material in another muscle fiber. Scale bar = 2.5 μm. (i) Perinuclear proliferation of membranous and tubular structures of presumed SR origin evolving into myelin-like autophagic material (black arrows) surrounding a non-subsarcolemmal myonucleus. White arrows: enlarged, swollen mitochondria. Scale bar = 4 μm. (j) Perinuclear SR membrane proliferation (black arrows). Scale bar = 1.5 μm. (k) Membrane outfolding (black arrow) continuous with the nuclear envelope. Scale bar = 0.4 μm. (l) Lobulated myonucleus surrounded by membrane proliferations and membranous (black arrow) and granular autophagic material. Scale bar = 1.5 μm. (m) Markedly condensed chromatin especially at the nuclear membrane in a pyknotic myonucleus. Scale bar = 2.0 μm. (n) Subsarcolemmal myonucleus showing chromatin condensation at the nuclear membrane accompanied by two perinuclear autophagic vacuoles. Scale bar = 7 μm. (PPT 3728 kb)
401_2013_1224_MOESM6_ESM.ppt
Suppl. Fig. 4 Ultrastructural alterations in wz mouse muscle at age 26 weeks analyzed by transmission electron microscopy. (a) Myonucleus showing chromatin condensation at the nuclear envelope. Black arrows: perinuclear SR tubule and membrane proliferation. (M): enlarged, swollen mitochondrion. Scale bar = 2.0 μm. (b) Prominently widened SR (black arrows). Scale bar = 2.5 μm. (c) Focal myonuclear lobulation (black arrows). (M): swollen mitochondria in an adjacent muscle fiber. Scale bar = 2.0 μm. (d) Late stage of myonuclear degeneration (black arrow). Scale bar = 3.0 μm. (e) Pronounced lobulation of a myonucleus. Scale bar = 2.0 μm. (f) Large subsarcolemmal autophagic vacuole containing myelin-like osmiophilic material. Scale bar = 2 μm. (g) Condensed muscle fiber mitochondrion (black arrow) engulfed by osmiophilic membranes, indicating mitophagia. Scale bar = 0.3 μm. (h) Large subsarcolemmal autophagic vacuole containing granular and membranous autophagic material. Black arrow: remanants of a myonucleus. Scale bar = 1.8 μm. (i) Autophagic vacuole containing myelin-like osmiophilic material. Scale bar = 2 μm. (j) Focal lift off of (leaflet of) the envelope (black arrows) of a myonucleus with markedly condensed chromatin. Scale bar = 0.8 μm. (k) Another myonucleus showing similar alterations of the envelope (black arrow). Scale bar = 1.8 μm. (l) Lobulated myonucleus with adjacent autophagic material (black arrow). Scale bar = 0.5 μm. (m) Centralized myonuclei one of which is showing pronounced chromatin condensation (black arrow). Scale bar = 4.0 μm. (PPT 2499 kb)
401_2013_1224_MOESM7_ESM.ppt
Suppl. Fig. 5 Ultrastructural alterations in wz mouse muscle at age 84 weeks. (a, b) Prominent focal widening of the SR (black arrows). Scale bar = 2.5 μm. (c, d) Focal mitochondrial swelling (black arrows). Scale bar = in (c) = 1 μm, in (d) 0.7 μm. (e-i) Membranous perinuclear autophagic material (black arrows). Scale bars = 1.2 μm in (e–g), 2.5 μm in (h), 1.5 μm in (i). (j-n) Lift-off, outfolding and extension of nuclear envelope (black arrows) of several degenerating myonuclei partially combined with marked proliferation of other membranous as well widened and proliferated tubular (SR) structures (white arrows). Scale bars in (j and k) = 4 μm, in (l) 3 μm, in (m and n) = 2 μm. (PPT 5972 kb)
401_2013_1224_MOESM8_ESM.ppt
Suppl. Fig. 6 Cerebellar alterations in wz mice. (a) Normal density of Purkinje cells (white arrows) and absence of glial cell proliferation in the wt littermate control. Scale bar = 40 μm. (b) Single remaining Purkinje cell (white arrow); black arrows: proliferated (Bergmann) glial cells in a 26-week-old wz mouse. Scale bar = 40 μm. (PPT 440 kb)
401_2013_1224_MOESM9_ESM.ppt
Suppl. Fig. 7 (e) Further analysis of selected proteins in three animal pairs at age 26 weeks. Presence of muscle fiber ER stress, activation of UPR and ERAD pathway as well as of lysosomal–autophagy could be confirmed by alterations in paradigmatic factors in the three pairs. GAPDH was used as loading control and as normalization factor for protein increase studies (upper bar graph). Results are consistent throughout all different ages of animals analyzed (16 weeks, 26 weeks, 52 weeks and 84 weeks). Quantification of the three independent experiments is shown. Y-axis shows fold of increase. X-axis shows proteins analyzed. Student′s unpaired t test was used. P values were as following: Atf6(uncleaved) = 0.0413*; Atf6(cleaved) = 0.0010***; Becn1 = 0,0094**; BiP = 0,0743; Chop = 0,0942; eif2α = 0,8722; peif2α = 0,0112*; Dnajb6 = 0,3678; Grp170 = 0,1122; Lc3-I = 0,0001***; Lc3-II = 0,9842; Lmna = 0,0956; Lmnb1 = 0,0201*; Pdi = 0,0939; Perk = 0.2269; pPerk = 0,1495; Rab11 = 0,0393*; Sec62 = 0,0246*; Tdp-43 = 0,0058**; Vcp = 0,0022**. * significant greater or lower than control value; ** very significant greater or lower than control value; *** extremely significant greater or lower than control value. Lower bar graph shows average ratios of protein activation (phosphorylation or cleavage) in wild-type and woozy animals with the age of 26 weeks. Quantification of the three independent experiments is shown. The Y-axis shows fold of protein activation. The X-axis shows proteins analyzed. The letter u signifies the uncleaved or unphosphorylated status of the protein, respectively. The letter c signifies the cleaved form of a protein whereas the letter p signifies the phosphorylated form of a protein. Students unpaired t test was used. P values were as following: wt Atf6(uncleaved/cleaved) = 0.0072**; wz Atf6(uncleaved/cleaved) = 0.0105*; wt eif2α/peif2α = 0.3605; wz eif2α/peif2α = 0.0029**; wt Perk/pPerk = 0.1217; wz Perk/pPerk = 0.0387*; wt LC3(uncleaved(I)/cleaved(II)) = 0.5312; wz LC3(uncleaved(I)/cleaved(II)) = 0.4393. (PPT 516 kb)
Rights and permissions
About this article
Cite this article
Roos, A., Buchkremer, S., Kollipara, L. et al. Myopathy in Marinesco–Sjögren syndrome links endoplasmic reticulum chaperone dysfunction to nuclear envelope pathology. Acta Neuropathol 127, 761–777 (2014). https://doi.org/10.1007/s00401-013-1224-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1224-4