Abstract
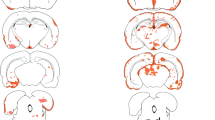
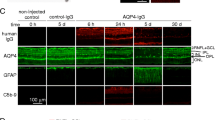
Animal models of neuromyelitis optica (NMO) are needed for elucidation of disease mechanisms and for testing new therapeutics. Prior animal models of NMO involved administration of human anti-aquaporin-4 immunoglobulin G antibody (NMO-IgG) to rats with pre-existing neuroinflammation, or to naïve mice supplemented with human complement. We report here the development of NMO pathology following passive transfer of NMO-IgG to naïve rats. A single intracerebral infusion of NMO-IgG to adult Lewis rats produced robust lesions around the needle track in 100 % of rats; at 5 days there was marked loss of aquaporin-4 (AQP4), glial fibrillary acidic protein (GFAP) and myelin, granulocyte and macrophage infiltration, vasculocentric complement deposition, blood–brain barrier disruption, microglial activation and neuron death. Remarkably, a distinct ‘penumbra’ was seen around lesions, with loss of AQP4 but not of GFAP or myelin. No lesions or penumbra were seen in rats receiving control IgG. The size of the main lesion with loss of myelin was greatly reduced in rats made complement-deficient by cobra venom factor or administered NMO-IgG lacking complement-dependent cytotoxicity (CDC) effector function. However, the penumbra was seen under these conditions, suggesting a complement-independent pathogenesis mechanism. The penumbra was absent with NMO-IgG lacking both CDC and antibody-dependent cellular cytotoxicity (ADCC) effector functions. Finally, lesion size was significantly reduced after macrophage depletion with clodronate liposomes. These results: (i) establish a robust, passive-transfer model of NMO in rats that does not require pre-existing neuroinflammation or complement administration; (ii) implicate ADCC as responsible for a unique type of pathology also seen in human NMO; and (iii) support a pathogenic role of macrophages in NMO.






Similar content being viewed by others
Abbreviations
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- AQP4:
-
Aquaporin-4
- CDC:
-
Complement-dependent cytotoxicity
- CDCC:
-
Complement-dependent cell-mediated cytotoxicity
- EAE:
-
Experimental autoimmune encephalomyelitis
- GFAP:
-
Glial fibrillary acidic protein
- MBP:
-
Myelin basic protein
- NMO:
-
Neuromyelitis optica
- NMO-IgG:
-
Neuromyelitis optica immunoglobulin G antibody
- WGA:
-
Wheat germ agglutinin
References
Aoyama M, Kakita H, Kato S, Tomita M, Asai K (2012) Region-specific expression of a water channel protein, aquaporin 4, on brain astrocytes. J Neurosci Res 90(12):2272–2280. doi:10.1002/jnr.23117
Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, Glogowska M, Case D, Antel JP, Owens GP, Gilden D, Nessler S, Stadelmann C, Hemmer B (2009) Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol 66(5):617–629. doi:10.1002/ana.21802
Bergman I, Basse PH, Barmada MA, Griffin JA, Cheung NK (2000) Comparison of in vitro antibody-targeted cytotoxicity using mouse, rat and human effectors. Cancer Immunol Immunother 49(4–5):259–266
Bodega G, Suarez I, Lopez-Fernandez LA, Almonacid L, Zaballos A, Fernandez B (2006) Possible implication of ciliary neurotrophic factor (CNTF) and beta-synuclein in the ammonia effect on cultured rat astroglial cells: a study using DNA and protein microarrays. Neurochem Int 48(8):729–738. doi:S0197-0186(06)00004-0
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23(2):297–308
Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS (2011) Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. J Biol Chem 286(18):16516–16524. doi:10.1074/jbc.M111.227298
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24(9):2143–2155. doi:10.1523/JNEUROSCI.3547-03.2004
Frigeri A, Gropper MA, Turck CW, Verkman AS (1995) Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci USA 92(10):4328–4331
Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS (1995) Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci 108(Pt 9):2993–3002
Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, Lennon VA (2007) Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 69(24):2221–2231. doi:01.WNL.0000289761.64862.ce
Jacob A, McKeon A, Nakashima I, Sato DK, Elsone L, Fujihara K, de Seze J (2013) Current concept of neuromyelitis optica (NMO) and NMO spectrum disorders. J Neurol Neurosurg Psychiatry 84(8):922–930. doi:10.1136/jnnp-2012-302310
Jarius S, Paul F, Franciotta D, Waters P, Zipp F, Hohlfeld R, Vincent A, Wildemann B (2008) Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol 4(4):202–214. doi:10.1038/ncpneuro0764
Jarius S, Wildemann B (2010) AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol 6(7):383–392. doi:10.1038/nrneurol.2010.72
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29(43):13435–13444. doi:10.1523/JNEUROSCI.3257-09.2009
Kinoshita M, Nakatsuji Y, Kimura T, Moriya M, Takata K, Okuno T, Kumanogoh A, Kajiyama K, Yoshikawa H, Sakoda S (2009) Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun 386(4):623–627. doi:10.1016/j.bbrc.2009.06.085
Kinoshita M, Nakatsuji Y, Kimura T, Moriya M, Takata K, Okuno T, Kumanogoh A, Kajiyama K, Yoshikawa H, Sakoda S (2010) Anti-aquaporin-4 antibody induces astrocytic cytotoxicity in the absence of CNS antigen-specific T cells. Biochem Biophys Res Commun 394(1):205–210. doi:10.1016/j.bbrc.2010.02.157
Kira J (2011) Autoimmunity in neuromyelitis optica and opticospinal multiple sclerosis: astrocytopathy as a common denominator in demyelinating disorders. J Neurol Sci 311(1–2):69–77. doi:10.1016/j.jns.2011.08.043
Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J (2009) The role of the anaphylatoxins in health and disease. Mol Immunol 46(14):2753–2766. doi:10.1016/j.molimm.2009.04.027
Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202(4):473–477. doi:jem.20050304
Levin ME, Jin JG, Ji RR, Tong J, Pomonis JD, Lavery DJ, Miller SW, Chiang LW (2008) Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain. Pain 137(1):182–201. doi:S0304-3959(07)00672-0
Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, Trebst C, Weinshenker B, Wingerchuk D, Parisi JE, Lassmann H (2002) A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 125(Pt 7):1450–1461
Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J (2009) Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15(5):545–552. doi:10.1038/nm.1960
Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, Takahashi T, Nakashima I, Takahashi H, Itoyama Y (2007) Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 130(Pt 5):1224–1234. doi:awm047
Misu T, Hoftberger R, Fujihara K, Wimmer I, Takai Y, Nishiyama S, Nakashima I, Konno H, Bradl M, Garzuly F, Itoyama Y, Aoki M, Lassmann H (2013) Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol 125(6):815–827. doi:10.1007/s00401-013-1116-7
Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17(1):171–180
Papadopoulos MC, Verkman AS (2012) Aquaporin 4 and neuromyelitis optica. Lancet Neurol 11(6):535–544. doi:10.1016/S1474-4422(12)70133-3
Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14(4):265–277. doi:10.1038/nrn3468
Phuan PW, Ratelade J, Rossi A, Tradtrantip L, Verkman AS (2012) Complement-dependent cytotoxicity in neuromyelitis optica requires aquaporin-4 protein assembly in orthogonal arrays. J Biol Chem 287(17):13829–13839. doi:10.1074/jbc.M112.344325
Ratelade J, Asavapanumas N, Ritchie AM, Wemlinger S, Bennett JL, Verkman AS (2013) Involvement of antibody-dependent cell-mediated cytotoxicity in inflammatory demyelination in a mouse model of neuromyelitis optica. Acta Neuropathol 126(5):699–709. doi:10.1007/s00401-013-1172-z
Ratelade J, Bennett JL, Verkman AS (2011) Evidence against cellular internalization in vivo of NMO-IgG, aquaporin-4, and excitatory amino acid transporter 2 in neuromyelitis optica. J Biol Chem 286(52):45156–45164. doi:10.1074/jbc.M111.297275
Ratelade J, Bennett JL, Verkman AS (2011) Intravenous neuromyelitis optica autoantibody in mice targets aquaporin-4 in peripheral organs and area postrema. PLoS ONE 6(11):e27412. doi:10.1371/journal.pone.0027412
Ratelade J, Zhang H, Saadoun S, Bennett JL, Papadopoulos MC, Verkman AS (2012) Neuromyelitis optica IgG and natural killer cells produce NMO lesions in mice without myelin loss. Acta Neuropathol 123(6):861–872. doi:10.1007/s00401-012-0986-4
Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, Mandler RN, Weinshenker BG, Pittock SJ, Wingerchuk DM, Lucchinetti CF (2007) Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130(Pt 5):1194–1205. doi:awl371
Saadoun S, Bridges LR, Verkman AS, Papadopoulos MC (2012) Paucity of natural killer and cytotoxic T cells in human neuromyelitis optica lesions. NeuroReport 23(18):1044–1047. doi:10.1097/WNR.0b013e32835ab480
Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC (2010) Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133(Pt 2):349–361. doi:10.1093/brain/awp309
Saadoun S, Waters P, MacDonald C, Bell BA, Vincent A, Verkman AS, Papadopoulos MC (2012) Neutrophil protease inhibition reduces neuromyelitis optica-immunoglobulin G-induced damage in mouse brain. Ann Neurol 71(3):323–333. doi:10.1002/ana.22686
Saadoun S, Waters P, Macdonald C, Bridges LR, Bell BA, Vincent A, Verkman AS, Papadopoulos MC (2011) T cell deficiency does not reduce lesions in mice produced by intracerebral injection of NMO-IgG and complement. J Neuroimmunol 235(1–2):27–32. doi:10.1016/j.jneuroim.2011.03.007
Schlesinger LS, Horwitz MA (1991) Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol 147(6):1983–1994
Tradtrantip L, Asavapanumas N, Verkman AS (2013) Therapeutic cleavage of anti-aquaporin-4 autoantibody in neuromyelitis optica by an IgG-selective proteinase. Mol Pharmacol 83(6):1268–1275. doi:10.1124/mol.113.086470
Tradtrantip L, Ratelade J, Zhang H, Verkman AS (2013) Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody. Ann Neurol 73(1):77–85. doi:10.1002/ana.23741
Tradtrantip L, Zhang H, Saadoun S, Phuan PW, Lam C, Papadopoulos MC, Bennett JL, Verkman AS (2012) Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann Neurol 71(3):314–322. doi:10.1002/ana.22657
Van Rooijen N, Sanders A (1994) Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Meth 174(1–2):83–93
Vogel CW, Fritzinger DC (2010) Cobra venom factor: structure, function, and humanization for therapeutic complement depletion. Toxicon 56(7):1198–1222. doi:10.1016/j.toxicon.2010.04.007
Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29(37):11511–11522. doi:10.1523/JNEUROSCI.1514-09.2009
Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV (2013) Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 33(31):12870–12886. doi:10.1523/JNEUROSCI.2121-13.2013
Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG (2007) The spectrum of neuromyelitis optica. Lancet Neurol 6(9):805–815. doi:S1474-4422(07)70216-8
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66(10):1485–1489
Zhang H, Bennett JL, Verkman AS (2011) Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann Neurol 70(6):943–954. doi:10.1002/ana.22551
Zhang H, Verkman AS (2013) Eosinophil pathogenicity mechanisms and therapeutics in neuromyelitis optica. J Clin Invest 123(5):2306–2316. doi:10.1172/JCI67554
Acknowledgments
This work was supported by grants EY13574, EB00415, DK35124, HL73856, DK86125 and DK72517 from the National Institutes of Health, and a grant from the Guthy-Jackson Charitable Foundation. We thank Dr. Jeffrey Bennett (Univ. Colorado Denver, Aurora, CO) for providing recombinant monoclonal NMO antibody and for Accelerated Cure (Waltham, MA) for providing human NMO sera.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asavapanumas, N., Ratelade, J. & Verkman, A.S. Unique neuromyelitis optica pathology produced in naïve rats by intracerebral administration of NMO-IgG. Acta Neuropathol 127, 539–551 (2014). https://doi.org/10.1007/s00401-013-1204-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1204-8