Abstract
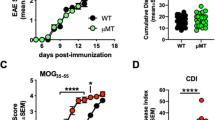
While the role of T cells has been studied extensively in multiple sclerosis (MS), the pathogenic contribution of B cells has only recently attracted major attention, when it was shown that B cell aggregates can develop in the meninges of a subset of MS patients and were suggested to be correlates of late-stage and more aggressive disease in this patient population. However, whether these aggregates actually exist has subsequently been questioned and their functional significance has remained unclear. Here, we studied myelin basic protein (MBP)–proteolipid protein (PLP)-induced experimental autoimmune encephalomyelitis (EAE), which is one of the few animal models for MS that is dependent on B cells. We provide evidence that B cell aggregation is reflective of lymphoid neogenesis in the central nervous system (CNS) in MBP–PLP-elicited EAE. B cell aggregation was present already few days after disease onset. With disease progression CNS B cell aggregates increasingly displayed the phenotype of tertiary lymphoid organs (TLOs). Our results further imply that these TLOs were not merely epiphenomena of the disease, but functionally active, supporting intrathecal determinant spreading of the myelin-specific T cell response. Our data suggest that the CNS is not a passive “immune-privileged” target organ, but rather a compartment, in which highly active immune responses can perpetuate and amplify the autoimmune pathology and thereby autonomously contribute to disease progression.







Similar content being viewed by others
Abbreviations
- cLN:
-
Cervical lymph node
- CNS:
-
Central nervous system
- drLN:
-
Draining lymph node
- EAE:
-
Experimental autoimmune encephalomyelitis
- FDC:
-
Follicular dendritic cell
- HEV:
-
High endothelial venule
- MAdCAM:
-
Mucosal addressin cell adhesion molecule
- MBP:
-
Myelin basic protein
- MOG:
-
Myelin oligodendrocyte glycoprotein
- MP4:
-
MBP–PLP fusion protein
- MS:
-
Multiple sclerosis
- OVA:
-
Ovalbumin
- PNAd:
-
Peripheral node addressin
- PLP:
-
Proteolipid protein
- TLO:
-
Tertiary lymphoid organ
References
Aloisi F, Pujol-Borrell R (2006) Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 6:205–217
Armengol MP, Cardoso-Schmidt CB, Fernández M, Ferrer X, Pujol-Borrell R, Juan M (2001) Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am J Pathol 159:861–873
Banks T, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenshki ML (1995) Lymphotoxin-alpha-deficient mice: effects on secondary lymphoid development and humoral immune responsiveness. J Immunol 155:1685–1693
Carragher DM, Rangel-Moreno J, Randall TD (2008) Ectopic lymphoid tissues and local immunity. Semin Immunol 20:26–42
De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J et al (1994) Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin alpha. Science 264:703–707
Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V, Caliqiuri G, Graff-Dubois S, Morelon E, Thaunat O (2010) Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol 184:5344–5351
Drayton DL, Liao S, Mounzer RH, Ruddle NH (2006) Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol 7:344–353
Hjelmström P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH (2000) Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol 156:1133–1138
Howell OW, Reeves CA, Nicholas R, Carassiti D, Radotra B, Gentleman SM, Serafini B, Aloisi F, Roncaroli F, Magliozzi R, Reynolds R (2011) Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134:2755–2771
Kooi E, Geurts JJG, van Horssen J, Bo L, van der Valk P (2009) Meningeal inflammation is not associated with cortical demyelination in chronic multiple sclerosis. J Neuropathol Exp Neurol 68:1021–1028
Kuerten S, Lichtenegger FS, Faas S, Angelov DN, Tary-Lehmann M, Lehmann PV (2006) MBP–PLP fusion protein-induced EAE in C57BL/6 mice. J Neuroimmunol 177:99–111
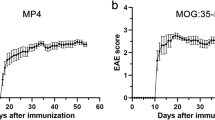
Kuerten S, Kostova-Bales DA, Frenzel LP, Tigno JT, Tary-Lehmann M, Angelov DN, Lehmann PV (2007) MP4- and MOG:35-55-induced EAE in C57BL/6 mice differentially targets brain, spinal cord and cerebellum. J Neuroimmunol 189:31–40
Kuerten S, Javeri S, Tary-Lehmann M, Lehmann PV, Angelov DN (2008) Fundamental differences in the dynamics of CNS lesion development and composition in MP4- and MOG peptide 35-55-induced experimental autoimmune encephalomyelitis. Clin Immunol 129:256–267
Kuerten S, Pauly R, Rottlaender R, Rodi M, Gruppe TL, Addicks K, Tary-Lehmann M, Lehmann PV (2011) Myelin-reactive antibodies mediate the pathology of MBP–PLP fusion protein MP4-induced EAE. Clin Immunol 140:54–62
Lehmann PV, Forsthuber T, Miller A, Sercarz EE (1992) Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 358:155–157
Levine GD, Rosai J (1978) Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol 9:495–515
Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Brück W, Parisi JE, Scheithauer BW, Gianinni C, Weigand SD, Mandrekar J, Ransohoff RM (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365:2188–2197
MacLennan ICM (1994) Germinal centers. Ann Rev Immunol 12:117–139
Magalhaes R, Stiehl P, Morawietz L, Berek C, Krenn V (2002) Morphological and molecular pathology of the B cell response in synovitis of rheumatoid arthritis. Virchows Arch 441:415–427
Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F (2004) Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol 148:11–23
Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F (2007) Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130:1089–1104
Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, Fitzgerald O, Bresnihan B, Caporali R, Montecucco C, Uquccioni M, Pitzalis C (2005) Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol 35:1347–1359
McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD (2005) Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 11:335–339
McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD (1995) Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med 182:75–85
Mooij P, de Wit HJ, Drexhage H (2001) An excess of dietary iodine accelerates the development of a thyroid-associated lymphoid tissue in autoimmune prone BB rats. Clin Immunol Immunopathol 69:189–198
Novotny-Gommert E (1977) A modification of the Bielschowsky-silverimpregnation to demonstrate reticulin fibres. Mikroskopie 33:342–352
Park CS, Choi YS (2005) How do follicular dendritic cells interact intimately with B cells in the germinal center? Immunology 114:2–10
Peferoen LAN, Lamers F, Lodder LNR, Gerritsen WH, Huitinga I, Melief J, Giovannoni G, Meier U, Hintzen RQ, Verjans GM, van Nierop GP, Vos W, Peferoen-Baert RM, Middeldorp JM, van der Valk P, Amor S (2010) Epstein Barr virus is not a characteristic feature in the central nervous system in established multiple sclerosis. Brain 133:e137
Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, Wucherpfennig K, Turley S, Carroll MC, Sobel RA, Bettelli E, Kuchroo VK (2011) Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 35:986–996
Rottlaender A, Villwock H, Addicks K, Kuerten S (2011) Neuroprotective role of fibroblast growth factor-2 in experimental autoimmune encephalomyelitis. Immunology 133:370–378
Ruddle NH (1999) Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res 19:119–125
Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmström P, Wahren-Herlenius M, Jonsson R (2003) Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthr Rheum 48:3187–3201
Serafini B, Rosicarelli B, Magliozzi R, Aloisi F (2004) Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14:164–174
Targoni OS, Baus J, Hofstetter HH, Hesse MD, Karulin AY, Boehm BO, Forsthuber TG, Lehmann PV (2001) Frequencies of neuroantigen-specific T cells in the central nervous system versus the immune periphery during the course of experimental allergic encephalomyelitis. J Immunol 166:4757–4764
Torkildsen O, Stansberg C, Angelskår SM, Kooi EJ, Geurts JJ, van der Valk P, Myhr KM, Steen VM, Bø L (2010) Upregulation of immunoglobulin-related genes in cortical sections from multiple sclerosis patients. Brain Pathol 20:720–729
Willis SN, Stadelmann C, Rodig SJ, Caron T, Gattenloehner S, Mallozzi SS, Roughan JE, Almendinger SE, Blewett MM, Brück W, Hafler DA, O’Connor KC (2009) Epstein–Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain 132:3318–3328
Zheng B, Ozen Z, Zhang X, De Silva S, Marinova E, Guo L, Wansley D, Huston DP, West MR, Han S (2005) CXCL13 neutralization reduces the severity of collagen-induced arthritis. Arthr Rheum 52:620–626
Acknowledgments
We thank Jolanta Kozlowski, Jil Pochmann and Esra Kücüksarioglan for help with the experiments. This work was supported by research grants from the German Research Foundation (DFG) (project KU 2760/2-1), the Köln Fortune Programm, the Imhoff-Stiftung, a Jaqueline Du Pré grant [grants to S.K.] and a grant from the National Multiple Sclerosis Society RG 4126-A-7 [N.H.R.]. A.S. and C.K contributed equally to this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuerten, S., Schickel, A., Kerkloh, C. et al. Tertiary lymphoid organ development coincides with determinant spreading of the myelin-specific T cell response. Acta Neuropathol 124, 861–873 (2012). https://doi.org/10.1007/s00401-012-1023-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-1023-3