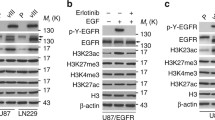
Abstract
The suppressor of cytokine signaling 3 (SOCS3) gene is one of eight structurally related genes of the SOCS family and has been suggested to function as a tumor suppressor by inhibition of the JAK/STAT signaling pathway. We investigated 60 human gliomas of different histological types for SOCS3 alterations and found frequent SOCS3 promoter hypermethylation and transcriptional downregulation. However, SOCS3 promoter hypermethylation was virtually absent in primary glioblastomas, which are characterized by frequent epidermal growth factor receptor (EGFR) amplification and overexpression. Assessment of the relationship between SOCS3 and EGFR aberrations revealed that SOCS3 promoter hypermethylation was inversely related to both the EGFR gene dosage as well as the EGFR protein expression, thus suggesting SOCS3 inactivation as a mechanism substituting for EGFR activation in a subset of gliomas. In support of this hypothesis, stable shRNA-mediated SOCS3 knock-down in U251 glioblastoma cells resulted in an activation of EGFR-related signaling pathways, i.e. an increase in the activation levels of STAT3, FAK and to a lesser extent MAPK, while the AKT phosphorylation levels remained unaffected. Functionally, SOCS3-depletion caused strongly increased tumor cell invasion with no obvious effect on tumor cell proliferation. In summary, our findings suggest that SOCS3 inactivation by promoter hypermethylation is mutually exclusive to EGFR activation in gliomas and preferentially promotes glioma cell invasion through STAT3 and FAK activation.





Similar content being viewed by others
References
Baltayiannis G, Baltayiannis N, Tsianos EV (2008) Suppressors of cytokine signaling as tumor repressors. Silencing of SOCS3 facilitates tumor formation and growth in lung and liver. J Buon 13:263–265
Barski D, Wolter M, Reifenberger G, Riemenschneider MJ (2010) Hypermethylation and transcriptional downregulation of the TIMP3 gene is associated with allelic loss on 22q12.3 and malignancy in meningiomas. Brain Pathol 20:623–631
Cooney RN (2002) Suppressors of cytokine signaling (SOCS): inhibitors of the JAK/STAT pathway. Shock 17:83–90
Fourouclas N, Li J, Gilby DC, Campbell PJ, Beer PA, Boyd EM, Goodeve AC, Bareford D, Harrison CN, Reilly JT, Green AR, Bench AJ (2008) Methylation of the suppressor of cytokine signaling 3 gene (SOCS3) in myeloproliferative disorders. Haematologica 93:1635–1644
He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, Costello JF, McCormick F, Jablons DM (2003) SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci USA 100:14133–14138
Imada K, Leonard WJ (2000) The Jak-STAT pathway. Mol Immunol 37:1–11
Kleihues P, Ohgaki H (1999) Primary and secondary glioblastomas: from concept to clinical diagnosis. Neurooncology 1:44–51
Krebs DL, Hilton DJ (2000) SOCS: physiological suppressors of cytokine signaling. J Cell Sci 113(Pt 16):2813–2819
Le Y, Zhu BM, Harley B, Park SY, Kobayashi T, Manis JP, Luo HR, Yoshimura A, Hennighausen L, Silberstein LE (2007) SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity 27:811–823
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Louis DN, Ohgaki H, Wiestler OD, CaveneeWK (eds) (2007) WHO Classification of tumours of the central nervous system, 4th edn. IARC Press, Lyon
Martini M, Pallini R, Luongo G, Cenci T, Lucantoni C, Larocca LM (2008) Prognostic relevance of SOCS3 hypermethylation in patients with glioblastoma multiforme. Int J Cancer 123:2955–2960
Mueller W, Nutt CL, Ehrich M, Riemenschneider MJ, von Deimling A, van den Boom D, Louis DN (2007) Downregulation of RUNX3 and TES by hypermethylation in glioblastoma. Oncogene 26:583–593
Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H (2005) Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene 24:6406–6417
Ohgaki H, Kleihues P (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170:1445–1453
Puhr M, Santer FR, Neuwirt H, Marcias G, Hobisch A, Culig Z (2010) SOCS-3 antagonises the proliferative and migratory effects of fibroblast growth factor-2 in prostate cancer by inhibition of p44/p42 MAPK signalling. Endocr Relat Cancer 17:525–538
Quesnelle KM, Boehm AL, Grandis JR (2007) STAT-mediated EGFR signaling in cancer. J Cell Biochem 102:311–319
Riemenschneider MJ, Jeuken JW, Wesseling P, Reifenberger G (2010) Molecular diagnostics of gliomas: state of the art. Acta Neuropathol 120:567–584
Riemenschneider MJ, Mueller W, Betensky RA, Mohapatra G, Louis DN (2005) In situ analysis of integrin and growth factor receptor signaling pathways in human glioblastomas suggests overlapping relationships with focal adhesion kinase activation. Am J Pathol 167:1379–1387
Riemenschneider MJ, Reifenberger G (2009) Molecular neuropathology of gliomas. Int J Mol Sci 10:184–212
Sasi W, Jiang WG, Sharma A, Mokbel K (2010) Higher expression levels of SOCS 1, 3, 4, 7 are associated with earlier tumour stage and better clinical outcome in human breast cancer. BMC Cancer 10:178
Seki E, Kondo Y, Iimuro Y, Naka T, Son G, Kishimoto T, Fujimoto J, Tsutsui H, Nakanishi K (2008) Demonstration of cooperative contribution of MET- and EGFR-mediated STAT3 phosphorylation to liver regeneration by exogenous suppressor of cytokine signalings. J Hepatol 48:237–245
Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA (2003) Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 278:13740–13746
Sutherland KD, Lindeman GJ, Choong DY, Wittlin S, Brentzell L, Phillips W, Campbell IG, Visvader JE (2004) Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene 23:7726–7733
Tepel M, Roerig P, Wolter M, Gutmann DH, Perry A, Reifenberger G, Riemenschneider MJ (2008) Frequent promoter hypermethylation and transcriptional downregulation of the NDRG2 gene at 14q11.2 in primary glioblastoma. Int J Cancer 123:2080–2086
Tokita T, Maesawa C, Kimura T, Kotani K, Takahashi K, Akasaka T, Masuda T (2007) Methylation status of the SOCS3 gene in human malignant melanomas. Int J Oncol 30:689–694
van den Boom J, Wolter M, Kuick R, Misek DE, Youkilis AS, Wechsler DS, Sommer C, Reifenberger G, Hanash SM (2003) Characterization of gene expression profiles associated with glioma progression using oligonucleotide-based microarray analysis and real-time reverse transcription-polymerase chain reaction. Am J Pathol 163:1033–1043
Weber A, Hengge UR, Bardenheuer W, Tischoff I, Sommerer F, Markwarth A, Dietz A, Wittekind C, Tannapfel A (2005) SOCS-3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene 24:6699–6708
Xia L, Wang L, Chung AS, Ivanov SS, Ling MY, Dragoi AM, Platt A, Gilmer TM, Fu XY, Chin YE (2002) Identification of both positive and negative domains within the epidermal growth factor receptor COOH-terminal region for signal transducer and activator of transcription (STAT) activation. J Biol Chem 277:30716–30723
Yang SF, Yeh YT, Wang SN, Hung SC, Chen WT, Huang CH, Chai CY (2008) SOCS-3 is associated with vascular invasion and overall survival in hepatocellular carcinoma. Pathology 40:558–563
Ying M, Li D, Yang L, Wang M, Wang N, Chen Y, He M, Wang Y (2010) Loss of SOCS3 expression is associated with an increased risk of recurrent disease in breast carcinoma. J Cancer Res Clin Oncol 136:1617–1626
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809
Yu ZB, Bai L, Qian P, Xiao YB, Wang GS, Qian GS, Bai CX, Min JX (2009) Restoration of SOCS3 suppresses human lung adenocarcinoma cell growth by downregulating activation of Erk1/2, Akt apart from STAT3. Cell Biol Int 33:995–1001
Zhou H, Miki R, Eeva M, Fike FM, Seligson D, Yang L, Yoshimura A, Teitell MA, Jamieson CA, Cacalano NA (2007) Reciprocal regulation of SOCS 1 and SOCS3 enhances resistance to ionizing radiation in glioblastoma multiforme. Clin Cancer Res 13:2344–2353
Acknowledgments
The authors would like to thank Nadine Lottmann (Department of Neuropathology, Düsseldorf) for skillful technical assistance. This work was financially supported by grants from the German Cancer Aid (Max-Eder Junior Research Group Program, Grant no. 107709 and 109426) and the Academy of Sciences of North Rhine-Westphalia/Mercator foundation (“Junges Kolleg”) (both to Markus J. Riemenschneider).
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Lindemann and O. Hackmann contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2011_832_MOESM1_ESM.jpg
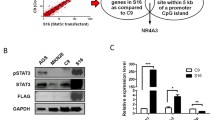
Suppl. Fig. 1. SOCS3 effect on tumor cell invasion after siRNA-mediated knock-down in U251 and A172 gioblastoma cells. (a) In comparison to the transfection with a scrambled negative control siRNA (CTRL), two different siRNAs per cell line were used to significantly downregulate SOCS3 expression levels relative to the ARF1 reference gene. (b) Note that tumor cell invasion significantly increases in both cell lines after siRNA-mediated SOCS3 knock-down in comparison to the transfection with respective negative control siRNAs. These findings corroborate the strong SOCS3 effect on glioma cell invasion observed in the stably transfected U251 knock-downclones. (JPEG 259 kb)
Rights and permissions
About this article
Cite this article
Lindemann, C., Hackmann, O., Delic, S. et al. SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathol 122, 241–251 (2011). https://doi.org/10.1007/s00401-011-0832-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0832-0