Abstract
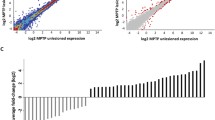
Prolonged treatment of Parkinson’s disease (PD) with levodopa leads to disabling side effects collectively referred to as ‘dyskinesias’. We hypothesized that bioenergetic function in the putamen might play a crucial role in the development of dyskinesias. To test this hypothesis, we used post mortem samples of the human putamen and applied real time–PCR approaches and gene expression microarrays. We found that mitochondrial DNA (mtDNA) levels are decreased in patients who have developed dyskinesias, and mtDNA damage is concomitantly increased. These pathologies were not observed in PD subjects without signs of dyskinesias. The group of nuclear mRNA transcripts coding for the proteins of the mitochondrial electron transfer chain was decreased in patients with dyskinesias to a larger extent than in patients who had not developed dyskinesias. To examine whether dopamine fluctuations affect mtDNA levels in dopaminoceptive neurons, rat striatal neurons in culture were repeatedly exposed to levodopa, dopamine or their metabolites. MtDNA levels were reduced after treatment with dopamine, but not after treatment with dopamine metabolites. Levodopa led to an increase in mtDNA levels. We conclude that mitochondrial susceptibility in the putamen plays a role in the development of dyskinesias.





Similar content being viewed by others
References
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458
Bender A, Krishnan KJ, Morris CM et al (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38:515–517
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 57:289–300
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Birkmayer W, Hornykiewicz O (1961) Der Dioxyphenylalanin (=DOPA)-Effekt bei der Parkinson-Akinese. Wien Klin Wochenschr 73:787–788
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193
Budd SL, Nicholls DG (1996) Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem 67:2282–2291
Bueler H (2009) Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp Neurol 218:235–246
Cenci MA, Lindgren HS (2007) Advances in understanding l-DOPA-induced dyskinesia. Curr Opin Neurobiol 17:665–671
Clay Montier LL, Deng JJ, Bai Y (2009) Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics 36:125–131
Cole RL, Konradi C, Douglass J, Hyman SE (1995) Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron 14:813–823
Cookson MR DJ-1, PINK1, and their effects on mitochondrial pathways. Mov Disord 25(Suppl 1):S44–S48
Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC (1992) Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet 2:324–329
Dennis G Jr, Sherman BT, Hosack DA et al (2003) DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4:P3
Dudman JT, Eaton ME, Rajadhyaksha A et al (2003) Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem 87:922–934
Fahn S, Oakes D, Shoulson I et al (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508
Garris PA, Ciolkowski EL, Pastore P, Wightman RM (1994) Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14:6084–6093
Gerfen CR (2000) Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci 23:S64–S70
Goto Y, Otani S, Grace AA (2007) The Yin and Yang of dopamine release: a new perspective. Neuropharmacology 53:583–587
Grace AA, Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4:2877–2890
Hattoria N, Wanga M, Taka H et al (2009) Toxic effects of dopamine metabolism in Parkinson’s disease. Parkinsonism Relat Disord 15(Suppl 1):S35–S38
Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
Jankovic J (2002) Levodopa strengths and weaknesses. Neurology 58:S19–S32
Jenner P (1993) Altered mitochondrial function, iron metabolism and glutathione levels in Parkinson’s disease. Acta Neurol Scand Suppl 146:6–13
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53(Suppl 3):S26–S36 (discussion S36–S28)
Kastner A, Anglade P, Bounaix C et al (1994) Immunohistochemical study of catechol-O-methyltransferase in the human mesostriatal system. Neuroscience 62:449–457
Konradi C (1998) The molecular basis of dopamine and glutamate interactions in the striatum. Adv Pharmacol 42:729–733
Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38:518–520
Laderman KA, Penny JR, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G (1996) Aging-dependent functional alterations of mitochondrial DNA (mtDNA) from human fibroblasts transferred into mtDNA-less cells. J Biol Chem 271:15891–15897
Li C, Wong WH (2001) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98:31–36
Maguire-Zeiss KA, Short DW, Federoff HJ (2005) Synuclein, dopamine and oxidative stress: co-conspirators in Parkinson’s disease? Brain Res Mol Brain Res 134:18–23
McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P (2001) Failure of the ubiquitin-proteasome system in Parkinson’s disease. Nat Rev Neurosci 2:589–594
Nicklas WJ, Saporito M, Basma A, Geller HM, Heikkila RE (1992) Mitochondrial mechanisms of neurotoxicity. Ann N Y Acad Sci 648:28–36
Pankratz N, Foroud T (2007) Genetics of Parkinson disease. Genet Med 9:801–811
Prithivirajsingh S, Story MD, Bergh SA et al (2004) Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett 571:227–232
Rajadhyaksha A, Barczak A, Macias W, Leveque JC, Lewis SE, Konradi C (1999) l-Type Ca(2+) channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons. J Neurosci 19:6348–6359
Rice ME, Cragg SJ (2008) Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev 58:303–313
Richfield EK, Penney JB, Young AB (1989) Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 30:767–777
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Schapira AH (2008) Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol 7:97–109
Schapira AH, Hartley A, Cleeter MW, Cooper JM (1993) Free radicals and mitochondrial dysfunction in Parkinson’s disease. Biochem Soc Trans 21:367–370
Schapira AH, Olanow CW (2008) Drug selection and timing of initiation of treatment in early Parkinson’s disease. Ann Neurol 64(Suppl 2):S47–S55
Yang JL, Weissman L, Bohr VA, Mattson MP (2008) Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair (Amst) 7:1110–1120
Zeevalk GD, Bernard LP, Song C, Gluck M, Ehrhart J (2005) Mitochondrial inhibition and oxidative stress: reciprocating players in neurodegeneration. Antioxid Redox Signal 7:1117–1139
Acknowledgments
The authors are indebted to the staff of the Harvard Brain Tissue Resource Center at McLean Hospital, who provided all tissues and to the study subjects. Angela Cenci, MD, provided helpful discussions. Melissa Hodges provided technical support in the laboratory. This work was supported by the National Institutes of Health [NS48235 to C.K.; MH068855 to HBTRC]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutes or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2010_740_MOESM1_ESM.doc
Demographic data of all samples. Not all samples were available for each experiment. Duration of disease and L-dopa levels were significantly different in the entire group. Thus, subgroups of samples were used which were controlled for these parameters. (DOC 121 kb)
401_2010_740_MOESM3_ESM.doc
Detailed gene expression data of electron transport proteins. ‘Mean’ data are natural values; p-values were obtained from log2 –transformed data to ensure normal distribution. (DOC 131 kb)
Rights and permissions
About this article
Cite this article
Naydenov, A.V., Vassoler, F., Luksik, A.S. et al. Mitochondrial abnormalities in the putamen in Parkinson’s disease dyskinesia. Acta Neuropathol 120, 623–631 (2010). https://doi.org/10.1007/s00401-010-0740-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0740-8