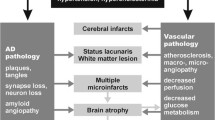
Abstract
The prevalence of Alzheimer disease (AD) and vascular dementia (VD) increases with advancing age, but less so after age 90 years. A retrospective hospital-based study of the relative prevalence of different disorders was performed in 1,110 consecutive autopsy cases of demented elderly in Vienna, Austria (66% females, MMSE <20; mean age 83.3 ± 5.4 SD years). It assessed clinical, general autopsy data and neuropathology including immunohistochemistry. Neuropathologic diagnosis followed current consensus criteria. Four age groups (7–10th decade) were evaluated. In the total cohort AD pathology was seen in 82.9% (“pure” AD 42.9%; AD + other pathologies 39.9%), VD in 10.8% (mixed dementia, MIX, i.e. AD + vascular encephalopathy in 5.5%); other disorders in 5.7%, and negative pathology in 0.8%. The relative prevalence of AD increased from age 60 to 89 years and decreased slightly after age 90+, while “pure” VD diagnosed in the presence of vascular encephalopathy of different types with low neuritic AD pathology (Braak stages <3; mean 1.2–1.6) decreased progressively from age 60 to 90+; 85–95% of these patients had histories of diabetes, morphologic signs of hypertension, 65% myocardial infarction/cardiac decompensation, and 75% a history of stroke(s). Morphologic subtypes, subcortical arteriosclerotic (the most frequent), multi-infarct encephalopathy, and strategic infarct dementia showed no age-related differences. The relative prevalence of AD + Lewy pathology remained fairly constant with increasing age. Mixed dementia and AD with minor cerebrovascular lesions increased significantly with age, while other dementias decreased. This retrospective study using strict morphologic criteria confirmed increased prevalence of AD with age, but mild decline at age 90+, and progressive decline of VD, while AD + vascular pathologies including MIX showed considerable age-related increase, confirming that mixed pathologies account for most dementia cases in very old persons. A prospective clinicopathologic study in oldest-old subjects showed a significant increase in both AD and cerebral amyloid angiopathy (CAA), but decrease in VD over age 85, while in a small group of old subjects CAA without considerable AD pathology may be an independent risk factor for cognitive decline.





Similar content being viewed by others
References
Aevarsson O, Skoog I (1996) A population-based study on the incidence of dementia disorders between 85 and 88 years of age. J Am Geriatr Soc 44:1455–1460
Akatsu H, Takahashi M, Matsukawa N, Ishikawa Y, Kondo N, Sato T, Nakazawa H, Yamada T, Okada H, Yamamoto T, Kosaka K (2002) Subtype analysis of neuropathologically diagnosed patients in a Japanese geriatric hospital. J Neurol Sci 196:63–69
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn. Text Revision (DSM-IV-TR). American Psychiatric Association, Washington, DC
Andin U, Gustafson L, Passant U, Brun A (2005) A clinicopathological study of heart and brain lesions in vascular dementia. Dement Geriatr Cogn Disord 19:222–228
Ankri J, Poupard M (2003) Prevalence and incidence of dementia among the very old: review of the literature. Rev Epidemiol Sante Publique 51:349–360
Attems J, Jellinger KA, Lintner F (2005) Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol 110:222–231
Attems J, Quass M, Jellinger KA, Lintner F (2007) Topographical distribution of cerebral amyloid angiopathy and its effect on cognitive decline are influenced by Alzheimer disease pathology. J Neurol Sci 257:49–55
Attems J, Lauda F, Jellinger KA (2008) Unexpectedly low prevalence of intracerebral hemorrhages in sporadic cerebral amyloid angiopathy: an autopsy study. J Neurol 255:70–76
Bacchetta JP, Kovari E, Merlo M, Canuto A, Herrmann FR, Bouras C, Gold G, Hof PR, Giannakopoulos P (2007) Validation of clinical criteria for possible vascular dementia in the oldest-old. Neurobiol Aging 28:579–585
Bancher C, Jellinger K, Lassmann H, Fischer P, Leblhuber F (1996) Correlations between mental state and quantitative neuropathology in the Vienna Longitudinal Study on Dementia. Eur Arch Psychiatry Clin Neurosci 246:137–146
Bancher C, Egensperger R, Kosel S, Jellinger K, Graeber MB (1997) Low prevalence of apolipoprotein E epsilon 4 allele in the neurofibrillary tangle predominant form of senile dementia. Acta Neuropathol 94:403–409
Berg L, McKeel DW Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM (1998) Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 55:326–335
Berlau DJ, Kahle-Wrobleski K, Head E, Goodus M, Kim R, Kawas C (2007) Dissociation of neuropathologic findings and cognition: case report of an apolipoprotein E epsilon2/epsilon2 genotype. Arch Neurol 64:1193–1196
Bermejo-Pareja F, Benito-Leon J, Vega S, Medrano MJ, Roman GC (2008) Incidence and subtypes of dementia in three elderly populations of central Spain. J Neurol Sci 264:63–72
Borjesson-Hanson A, Edin E, Gislason T, Skoog I (2004) The prevalence of dementia in 95 year olds. Neurology 63:2436–2438
Bowler JV (2005) Vascular cognitive impairment. J Neurol Neurosurg Psychiatry 76(Suppl 5):v35–v44
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, Paykel E, Mukaetova-Ladinska EB, Huppert FA, O’Sullivan A, Dening T, The Cambridge City Over-75 s Cohort Cc75c Study Neuropathology Collaboration (2009) Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge City over-75 s cohort (CC75C) study. J Alzheimers Dis 18:645–658
Bufill E, Bartes A, Moral A, Casadevall T, Codinachs M, Zapater E, Rovira JC, Perez R, Roura P, Blesa R (2009) Prevalence of cognitive deterioration in people over 80-years-old: COGMANLLEU study. Neurologia 24:102–107
Cadavid D, Mena H, Koeller K, Frommelt RA (2000) Cerebral beta amyloid angiopathy is a risk factor for cerebral ischemic infarction. A case control study in human brain biopsies. J Neuropathol Exp Neurol 59:768–773
Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, Mungas D, Reed BR, Kramer JH, Decarli CC, Weiner MW, Vinters HV (2006) Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol 60:677–687
Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease (1997) Neurobiol Aging 18:S1–S2
Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH (2008) Prevalence of dementia after age 90: results from the 90+ study. Neurology 71:337–343
Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB (2000) The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol 57:713–719
Crystal HA, Dickson D (2002) Cerebral infarcts in patients with autopsy proven Alzheimer’s disease (abstr.). Neurobiol Aging 23:207
del Ser T, Hachinski V, Merskey H, Munoz DG (2005) Alzheimer’s disease with and without cerebral infarcts. J Neurol Sci 231:3–11
Delaere P, He Y, Fayet G, Duyckaerts C, Hauw JJ (1993) Β A4 deposits are constant in the brain of the oldest old: an immunocytochemical study of 20 French centenarians. Neurobiol Aging 14:191–194
Duyckaerts C, Delatour B, Potier MC (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5–36
Ebly EM, Parhad IM, Hogan DB, Fung TS (1994) Prevalence and types of dementia in the very old: results from the Canadian Study of Health and Aging. Neurology 44:1593–1600
Esiri MM, Hyman BT, Beyreuther K, Masters CL (1997) Vascular dementia. In: Lantos P (ed) Greenfield’s neuropathology, 6th edn. Arnold Publishing, London, pp 204–210
Esiri MM, Wilcock GK, Morris JH (1997) Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry 63:749–753
Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD (1999) Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet 354:919–920
Fernando MS, Ince PG (2004) Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci 226:13–17
Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M (2005) Global prevalence of dementia: a Delphi consensus study. Lancet 366:2112–2117
Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JC, Jones B, Lyketsos C, Dulberg C (2004) Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 52:195–204
Fitzpatrick AL, Kuller LH, Lopez OL, Kawas CH, Jagust W (2005) Survival following dementia onset: Alzheimer’s disease and vascular dementia. J Neurol Sci 229–230:43–49
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Fratiglioni L, De Ronchi D, Aguero-Torres H (1999) Worldwide prevalence and incidence of dementia. Drugs Aging 15:365–375
Garcia JH, Brown GG (1992) Vascular dementia: neuropathologic alterations and metabolic brain changes. J Neurol Sci 109:121–131
Giannakopoulos P, Hof PR, Surini M, Michel JP, Bouras C (1993) Quantitative immunohistochemical analysis of the distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of nonagenarians and centenarians. Acta Neuropathol 85:602–610
Giannakopoulos P, Hof PR, Vallet PG, Giannakopoulos AS, Charnay Y, Bouras C (1995) Quantitative analysis of neuropathologic changes in the cerebral cortex of centenarians. Prog Neuropsychopharmacol Biol Psychiatry 19:577–592
Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E (2007) Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 130:2830–2836
Graves AB, Larson EB, Edland SD, Bowen JD, McCormick WC, McCurry SM, Rice MM, Wenzlow A, Uomoto JM (1996) Prevalence of dementia and its subtypes in the Japanese American population of King County, Washington state. The Kame Project. Am J Epidemiol 144:760–771
Greenberg SM, Gurol ME, Rosand J, Smith EE (2004) Amyloid angiopathy-related vascular cognitive impairment. Stroke 35:2616–2619
Grinberg LT, Ferretti RE, Leite REP, Farfel JM, Santos EB, Andrade MP, Alho ATL, do Canno Lima M, Oliveira KC, Caetano-Junior A, SE T, Polichiso L, Zoriki CS, Pasqualucci CAG, Jacob-Filho W, Nitrini R, BABS Group (2009) Vascular dementia, an avoidable disease, may be much more common than presumed: a postmortem study in a large population-based series (abs.). Alzheimers Dement 5(suppl.1):P164
Hall CB, Verghese J, Sliwinski M, Chen Z, Katz M, Derby C, Lipton RB (2005) Dementia incidence may increase more slowly after age 90: results from the Bronx Aging Study. Neurology 65:882–886
Hansen LA, Masliah E, Galasko D, Terry RD (1993) Plaque-only Alzheimer disease is usually the Lewy body variant, and vice versa. J Neuropathol Exp Neurol 52:648–654
Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP, Libow LS, Lesser GT, Maroukian M, Grossman HT (2008) Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol 65:1211–1217
Head E, Corrada MM, Kahle-Wrobleski K, Kim RC, Sarsoza F, Goodus M, Kawas CH (2009) Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol Aging 30:1125–1134
Hermann DM, Siccoli M, Brugger P, Wachter K, Mathis J, Achermann P, Bassetti CL (2008) Evolution of neurological, neuropsychological and sleep–wake disturbances after paramedian thalamic stroke. Stroke 39:62–68
Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM (1998) Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol 57:1168–1174
Ikeda K, Akiyama H, Arai T, Sahara N, Mori H, Usami M, Sakata M, Mizutani T, Wakabayashi K, Takahashi H (1997) A subset of senile dementia with high incidence of the apolipoprotein E epsilon2 allele. Ann Neurol 41:693–695
Imhof A, Kovari E, von Gunten A, Gold G, Rivara CB, Herrmann FR, Hof PR, Bouras C, Giannakopoulos P (2007) Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci 257:72–79
Jellinger KA, Bancher C (1998) Senile dementia with tangles (tangle predominant form of senile dementia). Brain Pathol 8:367–376
Jellinger KA (2001) Small concomitant cerebrovascular lesions are not important for cognitive decline in severe Alzheimer disease. Arch Neurol 58:520–521
Jellinger KA (2003) Plaque-predominant and tangle-predominant variants of Alzheimer’s disease. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press, Basel, pp 66–68
Jellinger KA (2005) Understanding the pathology of vascular cognitive impairment. J Neurol Sci 229–230:57–63
Jellinger KA, Attems J (2005) Prevalence and pathogenic role of cerebrovascular lesions in Alzheimer’s disease. J Neurol Sci 229–230:37–41
Jellinger KA (2006) Clinicopathological analysis of dementia disorders in the elderly: an update. J Alzheimers Dis 9:61–70
Jellinger KA, Attems J (2006) Prevalence and impact of cerebrovascular pathology in Alzheimer’s disease and parkinsonism. Acta Neurol Scand 114:38–46
Jellinger KA (2007) The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol 113:349–388
Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol 113:107–117
Jellinger KA, Attems J (2007) Neuropathological evaluation of mixed dementia. J Neurol Sci 257:80–87
Jellinger KA (2008) The pathology of “vascular dementia”: a critical update. J Alzheimers Dis 14:107–123
Jellinger KA, Attems J (2008) Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol 115:427–436
Jellinger KA (2009) Criteria for the neuropathological diagnosis of dementing disorders: routes out of the swamp? Acta Neuropathol 117:101–110
Jellinger KA, Attems J (2010) Tangel dominant dementia. In: Figueredo B, Meléndez F (eds) Neuroscience research advances. Nova Science Publishers Hauppauge, New York
Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T (2004) Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 226:75–80
Kase CS (1991) Epidemiology of multi-infarct dementia. Alzheimer Dis Assoc Disord 5:71–76
Kawas CH, Corrada MM (2006) Alzheimer’s and dementia in the oldest-old: a century of challenges. Curr Alzheimer Res 3:411–419
Keage HA, Carare RO, Friedland RP, Ince PG, Love S, Nicoll JA, Wharton SB, Weller RO, Brayne C (2009) Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC Neurol 9:3
Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, Greenberg SM (2009) Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology 72:1230–1235
Kliegel M, Moor C, Rott C (2004) Cognitive status and development in the oldest old: a longitudinal analysis from the Heidelberg Centenarian Study. Arch Gerontol Geriatr 39:143–156
Knopman DS, Rocca WA, Cha RH, Edland SD, Kokmen E (2002) Incidence of vascular dementia in Rochester, Minn, 1985–1989. Arch Neurol 59:1605–1610
Knopman DS, Parisi JE, Boeve BF, Cha RH, Apaydin H, Salviati A, Edland SD, Rocca WA (2003) Vascular dementia in a population-based autopsy study. Arch Neurol 60:569–575
Kobayashi M, Sato T, Sato A, Imamura T (2009) Oldest-old dementia in a Japanese memory clinic. Brain Nerve 61:972–978
Kuller LH, Lopez OL, Jagust WJ, Becker JT, DeKosky ST, Lyketsos C, Kawas C, Breitner JC, Fitzpatrick A, Dulberg C (2005) Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 64:1548–1552
Lee JH, Olichney JM, Hansen LA, Hofstetter CR, Thal LJ (2000) Small concomitant vascular lesions do not influence rates of cognitive decline in patients with Alzheimer disease. Arch Neurol 57:1474–1479
Leys D, Pasquier F, Parnetti L (1998) Epidemiology of vascular dementia. Haemostasis 28:134–150
Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, Copeland JR, Dartigues JF, Jagger C, Martinez-Lage J, Soininen H, Hofman A (2000) Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. neurologic diseases in the elderly research group. Neurology 54:S4–S9
Lopez Mongil R, Lopez Trigo JA, Castrodeza Sanz FJ, Tamames Gomez S, Leon Colombo T (2009) Prevalence of dementia in institutionalized patients: the RESYDEM study. Rev Esp Geriatr Gerontol 44:5–11
Masuda J, Tanaka K, Ueda K, Omae T (1988) Autopsy study of incidence and distribution of cerebral amyloid angiopathy in Hisayama, Japan. Stroke 19:205–210
Mayeux R (2008) Alzheimer’s disease: epidemiology. In: Duyckaerts C, Litvan I (eds) Handbook of clinical neurology, vol 89, 3rd edn. Elsevier, Edinburgh, pp 195–205
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872
Miklossy J (2003) Cerebral hypoperfusion induces cortical watershed microinfarcts which may further aggravate cognitive decline in Alzheimer’s disease. Neurol Res 25:605–610
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Mizutani T, Shimada H (1992) Neuropathological background of twenty-seven centenarian brains. J Neurol Sci 108:168–177
Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, Knopman D, Graff-Radford N, Geda Y, Lucas J, Kantarci K, Shiung M, Jack C, Silber M, Pankratz VS, Petersen R (2010) Mild cognitive impairment associated with limbic and neocortical lewy body disease: a clinicopathological study. Brain 133:540–556
Mura T, Dartigues J-F, Berr C (2010) How many dementia cases in France and Europe? Alternative projections and scenarios 2010–2050. Eur J Neurol 17:252–259
Perls T (2004) Centenarians who avoid dementia. Trends Neurosci 27:633–636
Perls T (2004) Dementia-free centenarians. Exp Gerontol 39:1587–1593
Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesberry W, Davis DG, Nelson J, Hardman J, Masaki KH, Vogt MR, Launer LJ, White LR (2005) AD lesions and infarcts in demented and no-demented Japanese-American men. Ann Neurol 57:98–103
Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ (2002) Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology 58:1629–1634
Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB (2007) Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 29:125–132
Polvikoski T, Sulkava R, Myllykangas L, Notkola IL, Niinisto L, Verkkoniemi A, Kainulainen K, Kontula K, Perez-Tur J, Hardy J, Haltia M (2001) Prevalence of Alzheimer’s disease in very elderly people: a prospective neuropathological study. Neurology 56:1690–1696
Polvikoski T, Sulkava R, Rastas S, Sutela A, Niinisto L, Notkola IL, Verkkoniemi A, Viramo P, Juva K, Haltia M (2006) Incidence of dementia in very elderly individuals: a clinical, neuropathological and molecular genetic study. Neuroepidemiology 26:76–82
Rahkonen T, Eloniemi-Sulkava U, Rissanen S, Vatanen A, Viramo P, Sulkava R (2003) Dementia with Lewy bodies according to the consensus criteria in a general population aged 75 years or older. J Neurol Neurosurg Psychiatry 74:720–724
Riekse RG, Leverenz JB, McCormick W, Bowen JD, Teri L, Nochlin D, Simpson K, Eugenio C, Larson EB, Tsuang D (2004) Effect of vascular lesions on cognition in Alzheimer’s disease: a community-based study. J Am Geriatr Soc 52:1442–1448
Riley KP, Snowdon DA, Markesbery WR (2002) Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol 51:567–577
Rocca WA, Hofman A, Brayne C, Breteler MM, Clarke M, Copeland JR, Dartigues JF, Engedal K, Hagnell O, Heeren TJ et al (1991) The prevalence of vascular dementia in Europe: facts and fragments from 1980 to 1990 studies: EURODEM Prevalence Research Group. Ann Neurol 30:817–824
Rockwood K, Ebly E, Hachinski V, Hogan D (1997) Presence and treatment of vascular risk factors in patients with vascular cognitive impairment. Arch Neurol 54:33–39
Sachdev PS, Chen X, Joscelyne A, Wen W, Altendorf A, Brodaty H (2007) Hippocampal size and dementia in stroke patients: the Sydney stroke study. J Neurol Sci 260:71–77
Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C (2009) Age, neuropathology, and dementia. N Engl J Med 360:2302–2309
Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69:2197–2204
Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA (2009) The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 18:691–701
Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66:200–208
Schoos BJ, Hansen LA, Alford MF, Tiraboschi P, Ho GJ, Thal LJ, Corey-Bloom J (1999) So much dementia, so little pathology: AD in the oldest old (abs.). Neurology 52:A479
Seno H, Ishino H, Inagaki T, Iijima M, Kaku K, Inata T (1999) A neuropathological study of dementia in nursing homes over a 17-year period, in Shimane Prefecture, Japan. Gerontology 45:44–48
Silver MH, Newell K, Brady C, Hedley-White ET, Perls TT (2002) Distinguishing between neurodegenerative disease and disease-free aging: correlating neuropsychological evaluations and neuropathological studies in centenarians. Psychosom Med 64:493–501
Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A (1993) A population-based study of dementia in 85-year-olds. N Engl J Med 328:153–158
Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ (2007) Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 62:406–413
Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R, Vinters HV (2010) Cerebral microinfarcts associated with severe cerebral β-amyloid angiopathy. Brain Pathol 20:459–467
Tanskanen M, Lindsberg PJ, Tienari PJ, Polvikoski T, Sulkava R, Verkkoniemi A, Rastas S, Paetau A, Kiuru-Enari S (2005) Cerebral amyloid angiopathy in a 95+ cohort: complement activation and apolipoprotein E (ApoE) genotype. Neuropathol Appl Neurobiol 31:589–599
Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru-Enari S, Paetau A, Tienari PJ, Myllykangas L (2008) Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in α2-macroglobulin and tau: a population-based autopsy study. Ann Med 40:232–239
Thal DR, Ghebremedhin E, Orantes M, Wiestler OD (2003) Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62:1287–1301
Thal DR, Griffin WS, de Vos RA, Ghebremedhin E (2008) Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathol 115:599–609
Thal DR, Capetillo-Zarate E, Larionov S, Staufenbiel M, Zurbruegg S, Beckmann N (2009) Capillary cerebral amyloid angiopathy is associated with vessel occlusion and cerebral blood flow disturbances. Neurobiol Aging 30:1936–1948
The-Canadian-Study, Group oHaAW (2000) The incidence of dementia in Canada. The Canadian Study of Health and Aging Working Group. Neurology 55:66–73
Tiraboschi P, Sabbagh MN, Hansen LA, Salmon DP, Merdes A, Gamst A, Masliah E, Alford M, Thal LJ, Corey-Bloom J (2004) Alzheimer disease without neocortical neurofibrillary tangles: “a second look”. Neurology 62:1141–1147
Tolnay M, Villoz N, Probst A, Miserez AR (2003) Apolipoprotein E genotype in senile dementia with tangles differs from Alzheimer’s disease. Neuropathol Appl Neurobiol 29:80–84
von Strauss E, Viitanen M, De Ronchi D, Winblad B, Fratiglioni L (1999) Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch Neurol 56:587–592
White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD, Hardman J, Davis D, Nelson J, Markesbery W (2005) Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol 18:224–227
White L (2009) Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia Aging Study. J Alzheimers Dis 18:713–725
Woodward M, Mackenzie IR, Feldman H (2006) High prevalence of multiple brain pathologies in dementia. Alzheimer’s Dement 2(Suppl1):S426
Xuereb JH, Brayne C, Dufouil C, Gertz H, Wischik C, Harrington C, Mukaetova-Ladinska E, McGee MA, O’Sullivan A, O’Connor D, Paykel ES, Huppert FA (2000) Neuropathological findings in the very old: results from the first 101 brains of a population-based longitudinal study of dementing disorders. Ann N Y Acad Sci 903:490–496
Yamada M, Tsukagoshi H, Otomo E, Hayakawa M (1987) Cerebral amyloid angiopathy in the aged. J Neurol 234:371–376
Zaccai J, Ince P, Brayne C (2006) Population-based neuropathological studies of dementia: design, methods and areas of investigation: a systematic review. BMC Neurol 6:2. doi:10.1186/1471-2377-1186-1182
Zekry D, Duyckaerts C, Moulias R, Belmin J, Geoffre C, Herrmann F, Hauw JJ (2002) Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol (Berl) 103:481–487
Zekry D, Duyckaerts C, Belmin J, Geoffre C, Moulias R, Hauw JJ (2003) Microvascular changes induced by cerebral amyloid angiopathy in the elderly: relationship with dementia. Acta Neuropathol 106:367–373
Zekry D, Herrmann FR, Grandjean R, Meynet MP, Michel JP, Gold G, Krause KH (2008) Demented versus non-demented very old inpatients: the same comorbidities but poorer functional and nutritional status. Age Ageing 37:83–89
Zhang ZX, Zahner GE, Roman GC, Liu J, Hong Z, Qu QM, Liu XH, Zhang XJ, Zhou B, Wu CB, Tang MN, Hong X, Li H (2005) Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol 62:447–453
Zhang ZX, Zahner GE, Roman GC, Liu XH, Wu CB, Hong Z, Hong X, Tang MN, Zhou B, Qu QM, Zhang XJ, Li H (2006) Socio-demographic variation of dementia subtypes in china: methodology and results of a prevalence study in Beijing, Chengdu, Shanghai, and Xian. Neuroepidemiology 27:177–187
Acknowledgments
The authors thank many colleagues from clinical departments and the Institute of Pathology, Otto Wagner Hospital, Vienna for clinical and autopsy data, and Mr. E. Mitter-Ferstl, PhD for secretarial and computer work. The study was supported by the Society for Support of Research in Experimental Neurology, Vienna, Austria.
Note added in proof
After termination of the present study, it was extended to a total of 1,700 demented elderly persons (671 male, 1,029 female, mean age at death 84.3 ± 6.0 years; autopsies 1981–2008), i.e. adding 590 autopsies, also divided into four age groups. The results were as follows: AD pathology was present in 80%, “pure” AD in 45.7% (36.9% Braak stage 5–6, 8.8% atypical forms), AD and other pathologies in 37.3%, VD in 12.8%, MIX in 5.2%, other degenerative dementias 4.1%, other disorders 1.6%, and negative pathology in 1.2%. The relative prevalence of AD increased from age 60 to 89 years from 38.9 to 48.9% and slightly decreased after age 90 (47.3%); MIX and AD + CVLs increased significantly from 5.2 to 10.6% and from 4.4 to 23.2% respectively (P < 0.001), while VD decreased from 15.0 to 8.7%. AD + LB pathology only slightly increased with age (7.8 to 9.4%), whereas other neurodegenerative disorders and other dementing diseases progressively decreased (21.0 to 0.4% and 5.0 to 0%, respectively). These data correlated well with those of the smaller autopsy series.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jellinger, K.A., Attems, J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol 119, 421–433 (2010). https://doi.org/10.1007/s00401-010-0654-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0654-5