Abstract
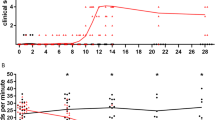
The involvement of dorsal root ganglia was studied in an in vivo model of experimental rabies virus infection using the challenge virus standard (CVS-11) strain. Dorsal root ganglia neurons infected with CVS in vitro show prolonged survival and few morphological changes, and are commonly used to study the infection. It has been established that after peripheral inoculation of mice with CVS the brain and spinal cord show relatively few neurodegenerative changes, but detailed studies of pathological changes in dorsal root ganglia have not previously been performed in this in vivo experimental model. In this study, adult ICR mice were inoculated in the right hindlimb footpad with CVS. Spinal cords and dorsal root ganglia were evaluated at serial time points for histopathological and ultrastructural changes and for biochemical markers of cell death. Light microscopy showed multifocal mononuclear inflammatory cell infiltrates in the sensory ganglia and a spectrum of degenerative neuronal changes. Ultrastructural changes in gangliocytes included features characteristic of the axotomy response, the appearance of numerous autophagic compartments, and aggregation of intermediate filaments, while the neurons retained relatively intact mitochondria and plasma membranes. Later in the process, there were more extensive degenerative neuronal changes without typical features of either apoptosis or necrosis. The degree of degenerative neuronal changes in gangliocytes contrasts with observations in CNS neurons in experimental rabies. Hence, gangliocytes exhibit selective vulnerability in this animal model. This contrasts markedly with the fact that they are, unlike CNS neurons, highly permissive to CVS infection in vitro. Further study is needed to determine mechanisms for this selective vulnerability, which will give us a better understanding of the pathogenesis of rabies.




Similar content being viewed by others
References
Buja LM, Eigenbrodt ML, Eigenbrodt EH (1993) Apoptosis and necrosis: basic types and mechanisms of cell death. Arch Pathol Lab Med 117:1208–1214
Castellanos J, Hurtado H, Arias J, Velandia A (1996) Rabies virus infection of cultured adult mouse dorsal root ganglion neurons. Mem Inst Oswaldo Cruz 91:621–625. doi:10.1590/S0074-02761996000500014
Castellanos JE, Martinez M, Acosta O, Hurtado H (2000) Nerve growth factor and neurotrophin-3 modulate the rabies infection of adult sensory neurons in primary cultures. Brain Res 871:120–126. doi:10.1016/S0006-8993(00)02408-2
Charlton KM, Casey GA (1979) Experimental rabies in skunks: immunofluorescence light and electron microscopic studies. Lab Invest 41:36–44
Debnath J, Baehrecke EH, Kroemer G (2005) Does autophagy contribute to cell death? Autophagy 1:66–74
Egan DA, Flumerfelt BA, Gwyn DG (1977) A light and electron microscopic study of axon reaction in the red nucleus of the rat following cervical and thoracic lesions. Neuropathol Appl Neurobiol 3:423–439. doi:10.1111/j.1365-2990.1977.tb00602.x
Eskelinen EL (2005) Maturation of autophagic vacuoles in mammalian cells. Autophagy 1:1–10
Eskelinen EL (2008) To be or not to be? Examples of incorrect identification of autophagic compartments in conventional transmission electron microscopy of mammalian cells. Autophagy 4:257–260
Fernyhough P, Smith DR, Schapansky J et al (2005) Activation of nuclear factor-κB via endogenous tumor necrosis factor α regulates survival of axotomized adult sensory neurons. J Neurosci 25:1682–1690. doi:10.1523/JNEUROSCI.3127-04.2005
Galluzzi L, Morselli E, Vicencio JM et al (2008) Life, death and burial: multifaceted impact of autophagy. Biochem Soc Trans 36:786–790. doi:10.1042/BST0360786
Hanlon CA, Niezgoda M, Rupprecht CE (2007) Rabies in terrestrial animals. In: Jackson AC, Wunner WH (eds) Rabies, 2 edn edn. Elsevier Academic Press, London, pp 201–258
Hanz S, Fainzilber M (2006) Retrograde signaling in injured nerve—the axon reaction revisited. J Neurochem 99:13–19. doi:10.1111/j.1471-4159.2006.04089.x
Havert MB, Schofield B, Griffin DE, Irani DN (2000) Activation of divergent neuronal cell death pathways in different target cell populations during neuroadapted Sindbis virus infection of mice. J Virol 74:5352–5356. doi:10.1128/JVI.74.11.5352-5356.2000
Hicks DJ, Nunez A, Healy DM, Brookes SM, Johnson N, Fooks AR (2009) Comparative pathological study of the murine brain after experimental infection with classical rabies virus and European bat lyssaviruses. J Comp Pathol 140:113–126
Jackson AC (2007) Human disease. In: Jackson AC, Wunner WH (eds) Rabies, 2nd edn. Elsevier Academic Press, London, pp 309–340
Jackson AC, Randle E, Lawrance G, Rossiter JP (2008) Neuronal apoptosis does not play an important role in human rabies encephalitis. J Neurovirol 14:368–375. doi:10.1080/13550280802216502
Johnson N, Mansfield KL, Hicks D et al (2008) Inflammatory responses in the nervous system of mice infected with a street isolate of rabies virus. In: Dodet B, Fooks AR, Müller T, Tordo N et al (eds) Towards the elimination of rabies in Eurasia. Kager, Basel, pp 65–72
Kelly RM, Strick PL (2000) Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods 103:63–71. doi:10.1016/S0165-0270(00)00296-X
Kroemer G, Galluzzi L, Vandenabeele P et al (2008) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11. doi:10.1038/cdd.2008.150
Kuzmin IV, Rupprecht CE (2007) Bat rabies. In: Jackson AC, Wunner WH (eds) Rabies, 2nd edn. Elsevier Academic Press, London, pp 259–307
Lieberman AR (1971) The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol 14:49–124. doi:10.1016/S0074-7742(08)60183-X
Lycke E, Tsiang H (1987) Rabies virus infection of cultured rat sensory neurons. J Virol 61:2733–2741
Mansfield KL, Johnson N, Nunez A, Hicks D, Jackson AC, Fooks AR (2008) Up-regulation of chemokine gene transcripts and T-cell infiltration into the central nervous system and dorsal root ganglia are characteristics of experimental European bat lyssavirus type 2 infection of mice. J Neurovirol 14:218–228. doi:10.1080/13550280802008297
Martinez-Gutierrez M, Castellanos JE (2007) Morphological and biochemical characterisation of sensory neurons infected in vitro with rabies virus. Acta Neuropathol 114:263–269. doi:10.1007/s00401-007-0222-9
Mitrabhakdi E, Shuangshoti S, Wannakrairot P et al (2005) Difference in neuropathogenetic mechanisms in human furious and paralytic rabies. J Neurol Sci 238:3–10. doi:10.1016/j.jns.2005.05.004
Morimoto K, Hooper DC, Spitsin S, Koprowski H, Dietzschold B (1999) Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J Virol 73:510–518
Murphy FA, Bauer SP, Harrison AK, Winn WC (1973) Comparative pathogenesis of rabies and rabies-like viruses: viral infection and transit from inoculation site to the central nervous system. Lab Invest 28:361–376
Park CH, Kondo M, Inoue S et al (2006) The histopathogenesis of paralytic rabies in six-week-old C57BL/6 J mice following inoculation of the CVS-11 strain into the right triceps surae muscle. J Vet Med Sci 68:589–595. doi:10.1292/jvms.68.589
Perl DP, Good PF (1991) The pathology of rabies in the central nervous system. In: Baer GM (ed) The natural history of rabies, 2nd edn. CRC Press, Boca Raton, Florida, pp 163–190
Rasalingam P, Rossiter JP, Jackson AC (2005) Recombinant rabies virus vaccine strain SAD-L16 inoculated intracerebrally in young mice produces a severe encephalitis with extensive neuronal apoptosis. Can J Vet Res 69:100–105
Rossiter JP, Jackson AC (2007) Pathology. In: Jackson AC, Wunner WH (eds) Rabies, 2nd edn. Elsevier Academic Press, London, pp 383–409
Scott CA, Rossiter JP, Andrew RD, Jackson AC (2008) Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome in experimental rabies in yellow fluorescent protein-expressing transgenic mice. J Virol 82:513–521. doi:10.1128/JVI.01677-07
Tang Y, Rampin O, Giuliano F, Ugolini G (1999) Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol 414:167–192. doi:10.1002/(SICI)1096-9861(19991115)414:2<167::AID-CNE3>3.0.CO;2-P
Tangchai P, Vejjajiva A (1971) Pathology of the peripheral nervous system in human rabies: a study of nine autopsy cases. Brain 94:299–306. doi:10.1093/brain/94.2.299
Tsiang H, Ceccaldi PE, Lycke E (1991) Rabies virus infection and transport in human sensory dorsal root ganglia neurons. J Gen Virol 72:1191–1194. doi:10.1099/0022-1317-72-5-1191
Van Gehuchten A, Nelis C (1900) Les lesions histologiques de la rage chez les animaux et chez l’homme. Bull Acad R Med Belg 14:31–66
Velandia ML, Perez-Castro R, Hurtado H, Castellanos JE (2007) Ultrastructural description of rabies virus infection in cultured sensory neurons. Mem Inst Oswaldo Cruz 102:441–447. doi:10.1590/S0074-02762007005000030
Weli SC, Scott CA, Ward CA, Jackson AC (2006) Rabies virus infection of primary neuronal cultures and adult mice: failure to demonstrate evidence of excitotoxicity. J Virol 80:10270–10273. doi:10.1128/JVI.01272-06
Acknowledgments
The authors are grateful for monoclonal antibody 5DF12 from Alexander I. Wandeler (Centre for Rabies Expertise, Canadian Food Inspection Agency, Nepean, Ontario). This work was supported by Canadian Institutes of Health Research Grant MOP-64376, and the Queen’s University Violet E. Powell Research Fund (all to A.C. Jackson). The authors wish to express their gratitude to Frederick A. Murphy (University of Texas Medical Branch, Galveston, TX, USA) for reviewing the electron micrographs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossiter, J.P., Hsu, L. & Jackson, A.C. Selective vulnerability of dorsal root ganglia neurons in experimental rabies after peripheral inoculation of CVS-11 in adult mice. Acta Neuropathol 118, 249–259 (2009). https://doi.org/10.1007/s00401-009-0503-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0503-6