Abstract
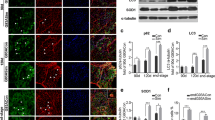
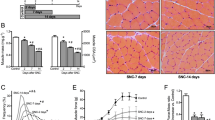
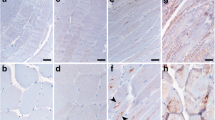
Chloroquine, an anti-malaria drug, is known to cause myopathy with rimmed vacuole formation. Although it disrupts the lysosomal degradation of proteins, the precise mechanism underlying muscle fiber degeneration has remained unclear. We investigated the temporal profiles of muscle fiber degeneration in chloroquine-treated rats, paying special attention to endoplasmic reticulum (ER) stress and autophagy. Male Wistar rats were intraperitoneally injected with chloroquine diphosphate at a dosage of 50 mg/kg body weight every day. We examined the localization and levels of proteins related to ER stress and autophagy in soleus muscle by means of immunohistochemistry and Western blotting at 3, 5, and 7 weeks after the beginning of the treatment. At 3 weeks, the levels of LC3-II and amyloid-β (Aβ) were increased. At 5 weeks, an unfolded protein response took place. At 7 weeks, rimmed vacuole formation became obvious. Interestingly, SERCA2, a Ca2+ -pump ATPase located in the endoplasmic/sarcoplasmic reticulum membrane was up-regulated at 5 weeks after treatment, but declined to the control level by 7 weeks. Taken together, these findings suggest that Aβ accumulation (at 3 weeks) caused by the disruption of lysosomal enzymes precedes an unfolded protein response (at 5 weeks). Next, activation of autophagy occurs (at 7 weeks), probably using sarcoplasmic reticulum membrane, the amount of which was increased. Chloroquine-treated rats could be useful for investigating the pathogenesis of diseases related to Aβ accumulation.





Similar content being viewed by others
References
Askanas V, McFerrin J, Alvarez RB et al (1997) Beta APP gene transfer into cultured human muscle induces inclusion-body myositis aspects. Neuroreport 8:2155–2158
Askanas V, Engel WK (2001) Inclusion body myositis: newest concepts of pathogenesis and relation to aging and Alzheimer disease. J Neuropathol Exp Neurol 60:1–14
Askanas V, Engel WK (2008) Inclusion body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer’s and Parkinson’s disease brains. Acta Neuropathol 116:583–595
Bence NF, Sampat RM, Kopito RR (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292:1552–1555
Bernales S, Schuck S, Walter P (2007) ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy 3:285–287
Butler D, Brown QB, Chin DJ et al (2005) Cellular responses to protein accumulation involve autophagy and lysosomal enzyme activation. Rejuvenation Res 8:227–237
Cardoso SM, Santana I, Swerdlow RH et al (2004) Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Aβ toxicity. J Neurochem 89:1417–1426
Caspersen C, Wang N, Yao J et al (2005) Mitochondrial Aβ: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J 19:2040–2041
Christensen RA, Shtifman A, Allen PD et al (2004) Calcium dyshomeostasis in beta-amyloid and tau-bearing skeletal myotubes. J Biol Chem 279:53524–53532
Du H, Guo L, Fang F et al (2008) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med 14:1097–1105
Fratta P, Engel WK, McFerrin J et al (2005) Proteasome inhibition and aggresome formation in sporadic inclusion-body myositis and in amyloid-beta precursor protein-overexpressing cultured human muscle fibers. Am J Pathol 167:517–526
Fukuchi K, Pham D, Hart M et al (1998) Amyloid-beta deposition in skeletal muscle of transgenic mice: possible model of inclusion body myopathy. Am J Pathol 153:1687–1693
Hamasaki M, Noda T, Baba M et al (2005) Starvation on triggers the delivery of the endoplasmic reticulum to the vacuole via autophagy in yeast. Traffic 6:56–65
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Hirai K, Aliev G, Nunomura A et al (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21:3017–3023
Ikezoe K, Furuya H, Ohyagi Y et al (2003) Dysferlin expression in tubular aggregates: their possible relationship to endoplasmic reticulum stress. Acta Neuropathol 105:603–609
Juhasz G, Neufeld TP (2006) Autophagy: a forty-year search for a missing membrane source. PLoS Biol 4:e36
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13:1211–1233
Kitazawa M, Green KN, Caccamo A et al (2006) Genetically augmenting Abeta42 levels in skeletal muscle exacerbates inclusion body myositis-like pathology and motor deficits in transgenic mice. Am J Pathol 168:1986–1997
Koh YH, von Arnim CA, Hyman BT et al (2005) BACE is degraded via the lysosomal pathway. J Biol Chem 280:32499–32504
Kouroku Y, Fujita E, Tanida I et al (2007) ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 14:230–239
Levine B, Yuan J (2005) Autophagy in cell death: an innocent convict? J Clin Invest 115:2679–2688
Lünemann JD, Schmidt J, Schmid D et al (2007) β-amyloid is a substrate of autophagy in sporadic inclusion body myositis. Ann Neurol 61:476–483
Macdonald RD, Engel AG (1970) Experimental chloroquine myopathy. J Neuropathol Exp Neurol 29:479–499
Malicdan MC, Noguchi S, Nonaka I et al (2007) A Gne knockout mouse expressing human GNE D167 V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Hum Mol Genet 16:2669–2682
Malhotra JD, Kaufman RJ (2007) The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18:716–731
Meusser B, Hirsch C, Jarasch E et al (2005) ERAD: the long road to destruction. Nat Cell Biol 7:766–772
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3:542–545
Mori K (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101:451–454
Nagaraju K, Casciola-Rosen L, Lundberg I et al (2005) Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfuncton. Arthritis Rheum 52:1824–1835
Nakagawa T, Zhu H, Morishima N et al (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Ogata M, Hino S, Saito A et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Pereira C, Ferreiro E, Cardoso SM et al (2004) Cell degeneration induced by amyloid-beta peptides: implications for Alzheimer’s disease. J Mol Neurosci 23:97–104
Schmalbruch H (1980) The early changes in experimental myopathy induced by chloroquine and chlorphentermine. J Neuropathol Exp Neurol 39:65–81
Seglen PO, Berg TO, Blankson H et al (1996) Structural aspects of autophagy. Adv Exp Med Biol 389:103–111
Stauber WT, Hedge AM, Trout JJ et al (1981) Inhibition of lysosomal function in red and white skeletal muscles by chloroquine. Exp Neurol 71:295–306
Suzuki T, Nakagawa M, Yoshikawa A et al (2002) The first molecular evidence that autophagy relates rimmed vacuole formation in chloroquine myopathy. J Biochem (Tokyo) 131:647–651
Tanida I, Minematsu-Ikeguchi N, Ueno T et al (2005) Lysosomal turnover, but not a cellular level, of endogeneous LC3 is a marker for autophagy. Autophagy 1:84–91
Tsuzuki K, Fukatsu R, Takamaru Y et al (1995) Amyloid beta protein in rat soleus muscle in chloroquine-induced myopathy using end-specific antibodies for A beta 40 and A beta 42: immunohistochemical evidence for amyloid beta protein. Neurosci Lett 202:77–80
Unterberger U, Hoftberger R, Gelpi E et al (2006) Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J Neuropathol Exp Neurol 65:348–357
Vattemi G, Engel WK, McFerrin J et al (2004) Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol 164:1–7
Yorimitsu T, Nair U, Yang Z et al (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Cell 281:30299–30304
Yoshida H, Matsui T, Yamamoto A et al (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891
Yu WH, Cuervo AM, Kumar A et al (2005) Macroautophagy—a novel beta-amyloid peptide generating pathway activated in Alzheimer’s disease. J Cell Biol 171:87–98
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikezoe, K., Furuya, H., Arahata, H. et al. Amyloid-β accumulation caused by chloroquine injections precedes ER stress and autophagosome formation in rat skeletal muscle. Acta Neuropathol 117, 575–582 (2009). https://doi.org/10.1007/s00401-009-0488-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0488-1