Abstract
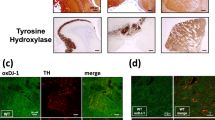
Glutathione peroxidase (GPx-1) is regarded as one of the mammalian cell’s main antioxidant enzymes inactivating hydrogen peroxide and protecting against oxidative stress. Using control, Parkinson’s disease (PD), and dementia with Lewy bodies tissue (DLB) we have shown that GPx-1 is a 21-kD protein under reducing conditions in all tissues examined but is not in high abundance in human brain. Using immunohistochemistry we have mapped the cellular distribution of GPx-1 and have shown it to be in highest levels in microglia and with lower levels in neurons. Only a trace amount was detectable in astrocytes using immunofluorescence and GPx-1 was not detectable in oligodendrocytes. GPx-1 positive microglia were hypertrophied and more abundant in PD and DLB tissues and were seen to be making multiple contacts with neurons. In some cases neurons containing Lewy bodies were surrounded by microglia. Unstructured Lewy bodies were enveloped with a layer of GPx-1 that was partially colocalized with α-synuclein whereas concentric Lewy bodies had discrete deposits of GPx-1 around the periphery which appeared to be involved in the degradation of the Lewy bodies. These results suggest that abnormal α-synuclein as found in Lewy bodies produce hydrogen peroxide and these neurons are capable of directing antioxidant enzymes to regions of oxidative stress. These results also suggest that GPx-1 positive microglia are involved in neuroprotection in PD and DLB and that GPx-1 is an important antioxidant enzyme in neuronal defences.






Similar content being viewed by others
References
Block ML, Hong J (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98. doi:10.1016/j.pneurobio.2005.06.004
Brigelius-Flohe R (1999) Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med 27:951–965. doi:10.1016/S0891-5849(99)00173-2
Crack PJ, Taylor JM, Flentjar NJ, de Haan J, Hertzog P, Iannello RC, Kola I (2001) Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem 78:1389–1399. doi:10.1046/j.1471-4159.2001.00535.x
Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F (1993) Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 52:1–6. doi:10.1016/0306-4522(93)90175-F
Deng Y, Lu J, Sivakumar V, Ling EA, Kaur C (2008) Amoeboid microglia in the periventricular white matter induce oligodendrocyte damage through expression of proinflammatory cytokines via MAP kinase signaling pathway hypoxic neonatal rats. Brain Pathol 18:387–400. doi:10.1111/j.1750-3639.2008.00138.x
Dringden R (2005) Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal 7:1223–1233. doi:10.1089/ars.2005.7.1223
Flentjar HJ, Crack PJ, Boyd R, Malin M, de Haan JB, Hertzog KolaI, Iannello R (2002) Mice lacking glutathione peroxidase-1 activity show increased tunel staining and an accelerated inflammatory response in brain following a cold-induced injury. Exp Neurol 177:9–20. doi:10.1006/exnr.2002.7927
Fukuhara R, Kageyama T (2003) Tissue distribution, molecular cloning, and gene expression of cytosolic glutathione peroxidase in Japanese monkey. Zoolog Sci 20:861–868. doi:10.2108/zsj.20.861
Gai QP, Yuan HX, Power JHT, Blumbergs PC, Jensen PH (2000) In situ and in vitro study of colocalization and segregation of α-synuclein, ubiquitin and lipids in Lewy bodies. Exp Neurol 166:324–333. doi:10.1006/exnr.2000.7527
Hashimoto M, Hse LJ, Xia Y, Takeda A, Sisk A, Sundsmo M, Masliah E (1999) Oxidative stress induces amyloid-like aggregate formation on NACP/α-synuclein in vitro. Neuroreport 10:717–721
Jahreiss L, Menzies FM, Rubinsztein DC (2008) The itinerary of autophagosomes: from peripheral formation to kiss-and-run fusion with lysosomes. Traffic 9:574–587. doi:10.1111/j.1600-0854.2008.00701.x
Kang JH, Kim KS (2003) Enhanced oligomerization of the α-synuclein mutant by the Cu, Zn-superoxide dismutase and hydrogen peroxide system. Mol Cells 15:87–93
Kim KS, Choi SY, Kwon HY, Won MH, Kang T, Kang JH (2002) Aggregation of α-synuclein induced by the Cu, Zn-superoxide dismutase and hydrogen peroxide system. Free Radic Biol Med 32:544–550. doi:10.1016/S0891-5849(02)00741-4
Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL (2002) Human α-synuclein-harboring familial Parkinson’s disease-linked Ala–53 → Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. Proc Natl Acad Sci USA 99:8968–8973. doi:10.1073/pnas.132197599
Lindenau J, Noack H, Asayama K, Wolf G (1998) Enhanced cellular glutathione peroxidase immunoreactivity in activated astrocytes and in microglia during excitotoxin induced neurodegeneration. Glia 24:252–256. doi:10.1002/(SICI)1098-1136(199810)24:2<252::AID-GLIA10>3.0.CO;2-Z
Liu CL, Chen S, Dietrich D, Hu BR (2008) Changes in autophagy after traumatic brain injury. J Cereb Blood Flow Metab 28:674–683. doi:10.1038/sj.jcbfm.9600587
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa d, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday g, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VMY, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier S, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies. Neurology 65:1863–1872. doi:10.1212/01.wnl.0000187889.17253.b1
Pandey N, Schmidt RE, Galvin JE (2006) The alpha-synuclein mutation E46K promotes aggregation in cultured cells. Exp Neurol 197:515–520. doi:10.1016/j.expneurol.2005.10.019
Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT (2005) Rotenone model of Parkinson’s disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem 280:42026–42035. doi:10.1074/jbc.M508628200
Power JHT, Asad S, Chataway T, Chegini F, Manavis J, Temlett JA, Jensen PH, Blumbergs PC, Gai W (2008) Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer’s disease pathology. Acta Neuropathol 115:611–622. doi:10.1007/s00401-008-0373-3
Power JHT, Shannon JM, Blumbergs PC, Gai W (2002) Nonselenium glutathione peroxidase in human brain: elevated levels in Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 161:885–894
Sikorska B, Liberski PP, Giraud P, Kopp N, Brown P (2004) Autophagy is part of ultrastructural synaptic pathology in Creutzfeldt-Jakob disease: a brain biopsy study. Int J Biochem Cell Biol 36:2563–2573. doi:10.1016/j.biocel.2004.04.014
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Huliman M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller d, Blancato J, Hardy J, Gwinn-Hardy K (2003) α-Synuclein locus triplication causes Parkinson’s disease. Science 302:841. doi:10.1126/science.1090278
Streit WJ, Graeber MB, Kreutzberg GW (1988) Functional plasticity of microglia: a review. Glia 1:301–307. doi:10.1002/glia.440010502
Streit WJ, Walter SA, Pennell NA (1999) Reactive microgliosis. Prog Neurobiol 57:563–581. doi:10.1016/S0301-0082(98)00069-0
Streit WJ (2005) Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev 48:234–239. doi:10.1016/j.brainresrev.2004.12.013
Tabner BJ, Turnbull S, El-Agnaf OMA, Allsop D (2002) Formation of hydrogen peroxide and hydroxyl radicals from Aβ and α-synuclein as a possible mechanism of cell death in Alzheimer’s disease and Parkinson’s disease. Free Radic Biol Med 32:1076–1083. doi:10.1016/S0891-5849(02)00801-8
Takizawa S, Matsushima K, Shinohara Y, Ogawa S, Komatsu N, Utsunomiya H, Watanabe K (1994) Immunohistochemical localization of glutathione peroxidase in infarcted human brain. J Neurol Sci 122:66–73. doi:10.1016/0022-510X(94)90053-1
Trepanier G, Furling D, Puymirat J, Mirault ME (1996) Immunocytochemical localization of seleno-glutathione peroxidase in the adult mouse brain. Neuroscience 75:231–243. doi:10.1016/0306-4522(96)00222-9
Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D (2001) Alpha-Synuclein implicated in Parkinson’s disease catalyses the formation of hydrogen peroxide in vitro. Free Radic Biol Med 30:1163–1170. doi:10.1016/S0891-5849(01)00513-5
Acknowledgments
We gratefully acknowledge the excellent assistance of the Flinders University Flinders University Microscopy and Image Analysis Facility and the South Australian Brain Bank. We also acknowledge the excellent technical assistance of Ms. Karen Humphreys and Ms. Christiane Simon. The useful discussions with Mr. James Manavis, Dr. Weiping Gai, and Professor James Temlett are gratefully acknowledged. This work was supported by the Flinders Medical Centre Foundation and a Flinders University Faculty Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Power, J.H.T., Blumbergs, P.C. Cellular glutathione peroxidase in human brain: cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol 117, 63–73 (2009). https://doi.org/10.1007/s00401-008-0438-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0438-3