Abstract
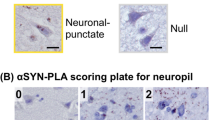
We immunohistochemically examined the neostriatum from 25 patients with symptomatic and presymptomatic Parkinson’s disease (PD) with various degrees of Lewy body pathology, using anti-phosphorylated α-synuclein (αS) antibody. These patients were classified according to the PD staging proposed by Braak et al. (Neurobiol Aging 24:197–211, 2003): stage II (αS pathology confined to the medulla oblongata and pontine tegmentum), stage III (αS pathology confined to the brainstem), stage IV (limbic stage), and stages V and VI (neocortical stage). αS immunohistochemistry revealed neuronal and glial cytoplasmic inclusions and neuritic changes in the neostriatum. αS inclusions were found in the medium-sized neurons (GABAergic neurons that project to the globus pallidus) and large neurons (cholinergic interneurons); the former began to appear at stage III and the latter was noted at stages V and VI. Neuritic changes and glial inclusions also began to appear at stage III. The numbers of neuronal and glial inclusions, and the extent of neuritic changes, correlated with the PD stage (P < 0.001). These findings suggest that intrinsic neostriatal neurons degenerate through αS aggregation during PD progression.



Similar content being viewed by others
References
Arai T, Ueda K, Ikeda K, Akiyama H, Haga C, Kondo H, Kuroki N, Niizato K, Iritani S, Tsuchiya K (1999) Argyrophilic glial inclusions in the midbrain of patients with Parkinson’s disease and diffuse Lewy body disease are immunopositive for NACP/α-synuclein. Neurosci Lett 259:83–86
Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T (1998) Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152:879–884
Bäckman CM, Shan L, Zhang Y, Hoffer BJ, Tomac AC (2007) Alterations in prodynorphin, proenkephalin, and GAD67 mRNA levels in the aged human putamen: correlation with Parkinson’s disease. J Neurosci Res 85:798–804
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Braak H, Bohl JR, Müller CM, Rüb U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051
Braak H, Sastre M, Del Tredici K (2007) Development of α-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol 114:231–241
Bugiani O, Perdelli F, Salvarani S, Leonardi A, Mancardi GL (1980) Loss of striatal neurons in Parkinson’s disease: a cytometric study. Eur Neurol 19:339–344
Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ (2002) Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol 52:205–210
Duda JE, Giasson BI, Mabon ME, Miller DC, Golbe LI, Lee VM, Trojanowski JQ (2002) Concurrence of α-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol 104:7–11
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164
Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW (2000) Distinctive neuropathology revealed by α-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol 99:663–672
Hishikawa N, Hashizume Y, Yoshida M, Sobue G (2001) Widespread occurrence of argyrophilic glial inclusions in Parkinson’s disease. Neuropathol Appl Neurobiol 27:362–372
Hishikawa N, Hashizume Y, Yoshida M, Sobue G (2003) Clinical and neuropathological correlates of Lewy body disease. Acta Neuropathol 105:341–350
Jellinger KA, Attems J (2006) Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol 112:253–260
Kimura H, McGeer PL, Peng F, McGeer EG (1980) Choline acetyltransferase-containing neurons in rodent brain demonstrated by immunohistochemistry. Science 208:1057–1059
Kosaka K (1978) Lewy bodies in cerebral cortex, report of three cases. Acta Neuropathol 42:127–134
Kotzbauer PT, Giasson BI, Kravitz AV, Golbe LI, Mark MH, Trojanowski JQ, Lee VM (2004) Fibrillization of α-synuclein and tau in familial Parkinson’s disease caused by the A53T α-synuclein mutation. Exp Neurol 187:279–288
Levy R, Herrero MT, Ruberg M, Villares J, Faucheux B, Guridi J, Guillen J, Luquin MR, Javoy-Agid F, Obeso JA et al (1995) Effects of nigrostriatal denervation and l-dopa therapy on the GABAergic neurons in the striatum in MPTP-treated monkeys and Parkinson’s disease: an in situ hybridization study of GAD67 mRNA. Eur J Neurosci 7:1199–1209
Lloyd KG, Mohler H, Heitz P, Bartholini G (1975) Distribution of choline acetyltransferase and glutamate decarboxylase within the substantia nigra and in other brain regions from control and Parkinsonian patients. J Neurochem 25:789–795
McGeer PL, McGeer EG, Wada JA, Jung E (1971) Effects of globus pallidus lesions and Parkinson’s disease on brain glutamic acid decarboxylase. Brain Res 32:425–431
McNeill TH, Brown SA, Rafols JA, Shoulson I (1988) Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res 455:148–152
Nishie M, Mori F, Fujiwara H, Hasegawa M, Yoshimoto M, Iwatsubo T, Takahashi H, Wakabayashi K (2004) Accumulation of phosphorylated α-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol 107:292–298
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 20:91–127
Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I (2005) α-Synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol 57:82–91
Piao YS, Wakabayashi K, Hayashi S, Yoshimoto M, Takahashi H (2000) Aggregation of α-synuclein/NACP in the neuronal and glial cells in diffuse Lewy body disease: a survey of six patients. Clin Neuropathol 19:163–169
Rinne JO, Laihinen A, Lonnberg P, Marjamaki P, Rinne UK (1991) A post-mortem study on striatal dopamine receptors in Parkinson’s disease. Brain Res 556:117–122
Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, Arai T, Nagura H, Yamanouchi H, Hasegawa M, Iwatsubo T, Murayama S (2003) Accumulation of phosphorylated α-synuclein in aging human brain. J Neuropathol Exp Neurol 62:644–654
Shoji M, Harigaya Y, Sasaki A, Ueda K, Ishiguro K, Matsubara E, Watanabe M, Ikeda M, Kanai M, Tomidokoro Y, Shizuka M, Amari M, Kosaka K, Nakazato Y, Okamoto K, Hirai S (2000) Accumulation of NACP/α-synuclein in Lewy body disease and multiple system atrophy. J Neurol Neurosurg Psychiatry 68:605–608
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) α-Synuclein in Lewy bodies. Nature 388:839–840
Takao M, Ghetti B, Yoshida H, Piccardo P, Narain Y, Murrell JR, Vidal R, Glazier BS, Jakes R, Tsutsui M, Spillantini MG, Crowther RA, Goedert M, Koto A (2004) Early-onset dementia with Lewy bodies. Brain Pathol 14:137–147
Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E (1998) Abnormal accumulation of NACP/α-synuclein in neurodegenerative disorders. Am J Pathol 152:367–372
Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H (1998) Accumulation of α-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol 96:445–452
Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H (2000) NACP/α-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol 99:14–20
Yamaguchi K, Cochran EJ, Murrell JR, Polymeropoulos MH, Shannon KM, Crowther RA, Goedert M, Ghetti B (2005) Abundant neuritic inclusions and microvacuolar changes in a case of diffuse Lewy body disease with the A53T mutation in the α-synuclein gene. Acta Neuropathol 110:298–305
Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ (2005) Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology 64:545–547
Zaja-Milatovic S, Keene CD, Montine KS, Leverenz JB, Tsuang D, Montine TJ (2006) Selective dendritic degeneration of medium spiny neurons in dementia with Lewy bodies. Neurology 66:1591–1593
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to K.W.), a grant from the Research Committee on Neurodegenerative Diseases, Ministry of Health, Labor and Welfare, Japan (to H.T.), and the Karoji Memorial Fund for Medical Research (to K.T.). The authors wish to express their gratitude to M. Nakata for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mori, F., Tanji, K., Zhang, H. et al. α-Synuclein pathology in the neostriatum in Parkinson’s disease. Acta Neuropathol 115, 453–459 (2008). https://doi.org/10.1007/s00401-007-0316-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0316-4