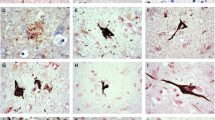
Abstract
The tau protein, well known as the primary component of neurofibrillary tangles, also comprises the Pick bodies found in Pick’s disease (PiD) and the glial lesions associated with progressive supranuclear palsy (PSP) and cortico-basal ganglionic degeneration (CBD). Many of the tau alterations that are characteristic of Alzheimer’s disease have also been identified in PSP and CBD. In this report, we examine three non-AD tauopathies (PSP, CBD, and PiD) for the presence of two specific tau alterations, phosphorylation at Ser422 and truncation at Asp421. We find that truncation at Asp421 is an alteration that is unique to neuronal lesions, occurring in Pick bodies as well as in neurofibrillary tangles, but not in lesions associated with glia. Conversely, phosphorylation at Ser422 is not only present in all these lesions, but identifies additional glial and neuronal pathology in disease-susceptible cortical regions. These results suggest that the molecular alterations of tau that occur during the initial process of tangle formation in AD are similar in non-AD tauopathies, but the middle and later changes are not common to all diseases.




Similar content being viewed by others
Abbreviations
- PSP:
-
Progressive supranuclear palsy
- CBD:
-
Cortico-basal ganglionic degeneration
- AD:
-
Alzheimer’s disease
- PiD:
-
Pick’s disease
- NFT:
-
Neurofibrillary tangles
References
Arai T, Ikeda K, Akiyama H, Tsuchiya K, Iritani S, Ishiguro K, Yagishita S, Oda T, Odawara T, Iseki E (2003) Different immunoreactivities of the microtubule-binding region of tau and its molecular basis in brains from patients with Alzheimer’s disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 105:489–498
Armstrong RA, Cairns NJ, Lantos PL (1999) Laminar distribution of Pick bodies, Pick cells and Alzheimer disease pathology in the frontal and temporal cortex in Pick’s disease. Neuropathol Appl Neurobiol 25:266–271
Berry RW, Sweet AP, Clark FA, Lagalwar S, Lapin BR, Wang T, Topgi S, Guillozet-Bongaarts AL, Cochran EJ, Bigio EH, Binder LI (2004) Tau epitope display in progressive supranuclear palsy and corticobasal degeneration. J Neurocytol 33:287–295
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278
Buee-Scherrer V, Condamines O, Mourton-Gilles C, Jakes R, Goedert M, Pau B, Delacourte A (1996) AD2, a phosphorylation-dependent monoclonal antibody directed against tau proteins found in Alzheimer’s disease. Brain Res Mol Brain Res 39:79–88
Carmel G, Mager EM, Binder LI, Kuret J (1996) The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem 271:32789–32795
Dickson DW (2001) Neuropathology of Pick’s disease. Neurology 56:16S–20S
Feany MB, Ksiezak-Reding H, Liu WK, Vincent I, Yen SH, Dickson DW (1995) Epitope expression and hyperphosphorylation of tau protein in corticobasal degeneration: differentiation from progressive supranuclear palsy. Acta Neuropathol 90:37–43
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 100:10032–10037
Garcia-Sierra F, Wischik CM, Harrington CR, Luna-Munoz J, Mena R (2001) Accumulation of C-terminally truncated tau protein associated with vulnerability of the perforant pathway in early stages of neurofibrillary pathology in Alzheimer’s disease. J Chem Neuroanat 22:65–77
Garcia-Sierra F, Ghoshal N, Quinn B, Berry RW, Binder LI (2003) Conformational changes and truncation of tau protein during tangle evolution in Alzheimer’s disease. J Alzheimers Dis 5:65–77
Garcia-Sierra F, Berry RW, Lagalwar S, Ghoshal N, Quinn B, Cryns VL, Binder LI (2002) Cleavage of tau protein at Asp-421 caspase site and truncation at Glu-391 during the formation and evolution of neurofibrillary tangles. 2002 Abstract Viewer/Itinerary Planner, Society for Neuroscience
Ghoshal N, Garcia-Sierra F, Fu Y, Beckett LA, Mufson EJ, Kuret J, Berry RW, Binder LI (2001) Tau-66: evidence for a novel tau conformation in Alzheimer’s disease. J Neurochem 77:1372–1385
Goedert M, Jakes R, Vanmechelen E (1995) Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 189:167–169
Graham D, Lantos P (eds) (2002) Greenfield’s neuropathology, 7th edn. Arnold, London
Guillozet-Bongaarts AL, Cahill ME, Cryns VL, Reynolds MR, Berry RW, Binder LI (2006) Pseudophosphorylation of tau at serine422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem 97:1005–1014
Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI (2005) Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging 26:1015–1022
Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI (2004) Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci 24:7895–7902
Jellinger KA, Stadelmann C (2000) Mechanisms of cell death in neurodegenerative disorders. J Neural Transm Suppl 59:95–114
Jicha GA, Bowser R, Kazam IG, Davies P (1997) Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res 48:128–132
Newman J, Rissman RA, Sarsoza F, Kim RC, Dick M, Bennett DA, Cotman CW, Rohn TT, Head E (2005) Caspase-cleaved tau accumulation in neurodegenerative diseases associated with tau and alpha-synuclein pathology. Acta Neuropathol 110:135–144
Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114:121–130
Su JH, Cummings BJ, Cotman CW (1994) Early phosphorylation of tau in Alzheimer’s disease occurs at Ser-202 and is preferentially located within neurites. Neuroreport 5:2358–2362
Su JH, Cummings BJ, Cotman CW (1996) Plaque biogenesis in brain aging and Alzheimer’s disease. I. Progressive changes in phosphorylation states of paired helical filaments and neurofilaments. Brain Res 739:79–87
Su JH, Nichol KE, Sitch T, Sheu P, Chubb C, Miller BL, Tomaselli KJ, Kim RC, Cotman CW (2000) DNA damage and activated caspase-3 expression in neurons and astrocytes: evidence for apoptosis in frontotemporal dementia. Exp Neurol 163:9–19
Uboga NV, Price JL (2000) Formation of diffuse and fibrillar tangles in aging and early Alzheimer’s disease. Neurobiol Aging 21:1–10
Wischik CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA (1988) Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA 85:4884–4888
Wischik CM, Novak M, Thogersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A (1988) Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA 85:4506–4510
Yang L, Ksiezak-Reding H (1998) Ubiquitin immunoreactivity of paired helical filaments differs in Alzheimer’s disease and corticobasal degeneration. Acta Neuropathol 96:520–526
Yoshimura N (1989) Topography of Pick body distribution in Pick’s disease: a contribution to understanding the relationship between Pick’s and Alzheimer’s diseases. Clin Neuropathol 8:1–6
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is supported by grants AG021184 and AG09466 from the NIH.
Rights and permissions
About this article
Cite this article
Guillozet-Bongaarts, A.L., Glajch, K.E., Libson, E.G. et al. Phosphorylation and cleavage of tau in non-AD tauopathies. Acta Neuropathol 113, 513–520 (2007). https://doi.org/10.1007/s00401-007-0209-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0209-6