Abstract
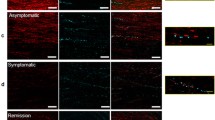
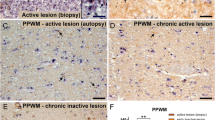
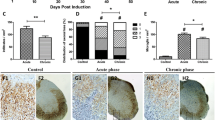
Axonal degeneration contributes to the transient and permanent neurological deficits seen in multiple sclerosis, an inflammatory disease of the central nervous system. To study the immunological mechanisms causing axonal degeneration, we induced experimental autoimmune encephalomyelitis (EAE) in wildtype Lewis rats and Lewis rats with a slowly progressive myelin degeneration due to proteolipid protein (PLP) overexpression. EAE was triggered either by the transfer of encephalitogenic T-cells alone or by the co-transfer of T-cells with demyelinating antibodies. Inducible nitric oxide synthase (iNOS) expression in perivascular macrophages was associated with a transient functional disturbance of axons, reflected by the focal and reversible accumulation of amyloid precursor protein. Clinical disease correlated with the numbers of APP positive axon spheroids. Demyelination was associated with a further increase of iNOS expression in macrophages and with a higher degree of axonal injury. Our studies suggest that nitric oxide and its metabolites contribute to axonal pathology and possibly also to subsequent neurological dysfunction in EAE.






Similar content being viewed by others
Abbreviations
- MOG:
-
Myelin oligodendrocyte glycoprotein
- α-MOG:
-
Anti-MOG antibodies
- APP:
-
Amyloid precursor protein
- CNP:
-
2′3′-cyclic nucleotide 3′ phosphodiesterase
- EAE:
-
Experimental autoimmune encephalomyelitis
- GFAP:
-
Glial fibrillary acidic protein
- MBP:
-
Myelin basic protein
- NS:
-
Normal serum
- NT:
-
Nitrotyrosine
- PFA:
-
Paraformaldehyde
- PLP:
-
Proteolipid protein
- Tg:
-
Transgenics
References
Aboul-Enein F, Bauer J, Klein M, Schubart A, Flugel A, Ritter T, Kawakami N, Siedler F, Linington C, Wekerle H, Lassmann H, Bradl M (2004) Selective and antigen-dependent effects of myelin degeneration on central nervous system inflammation. J Neuropathol Exp Neurol 63:1284–1296
Bauer J, Bradl M, Klein M, Leisser M, Deckwerth TL, Wekerle H, Lassmann H (2002) Endoplasmic reticulum stress in PLP-overexpressing transgenic rats: gray matter oligodendrocytes are more vulnerable than white matter oligodendrocytes. J Neuropathol Exp Neurol 61:12–22
Beckman JS (2002) Protein tyrosine nitration and peroxynitrite. FASEB J 16:1144
Ben Nun A, Wekerle H, Cohen IR (1981) The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol 11:195–199
Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W (2000) Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123:1174–1183
Blight AR (1993) Remyelination, revascularization, and recovery of function in experimental spinal cord injury. Adv Neurol 59:91–104
Bradl M, Bauer J, Inomata T, Zielasek J, Nave KA, Toyka K, Lassmann H, Wekerle H (1999) Transgenic Lewis rats overexpressing the proteolipid protein gene: myelin degeneration and its effect on T cell-mediated experimental autoimmune encephalomyelitis. Acta Neuropathol 97:595–606
Chalk JB, McCombe PA, Pender MP (1994) Conduction abnormalities are restricted to the central nervous system in experimental autoimmune encephalomyelitis induced by inoculation with proteolipid protein but not with myelin basic protein. Brain 117:975–986
Charcot JM (1868) Histologie de la sclerose en plaque. Gaz Hosp (Paris) 41:554–556
Cross AH, Manning PT, Stern MK, Misko TP (1997) Evidence for the production of peroxynitrite in inflammatory CNS demyelination. J Neuroimmunol 80:121–130
De Groot CJ, Ruuls SR, Theeuwes JW, Dijkstra CD, Van der Valk P (1997) Immunocytochemical characterization of the expression of inducible and constitutive isoforms of nitric oxide synthase in demyelinating multiple sclerosis lesions. J Neuropathol Exp Neurol 56:10–20
Diestel A, Aktas O, Hackel D, Hake I, Meier S, Raine CS, Nitsch R, Zipp F, Ullrich O (2003) Activation of microglial poly(ADP-ribose)-polymerase-1 by cholesterol breakdown products during neuroinflammation: a link between demyelination and neuronal damage. J Exp Med 198:1729–1740
Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA (1995) Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol 155:5769–5776
Garbern JY, Yool DA, Moore GJ, Wilds IB, Faulk MW, Klugmann M, Nave KA, Sistermans EA, van der Knaap MS, Bird TD, Shy ME, Kamholz JA, Griffiths IR (2002) Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 125:551–561
Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave KA (1998) Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280:1610–1613
Hoftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, Lassmann H (2004) Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol 14:43–50
Hooper DC, Bagasra O, Marini JC, Zborek A, Ohnishi ST, Kean R, Champion JM, Sarker AB, Bobroski L, Farber JL, Akaike T, Maeda H, Koprowski H (1997) Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. PNAS 94:2528–2533
Kapoor R, Davies M, Smith KJ (1999) Temporary axonal conduction block and axonal loss in inflammatory neurological disease: a potential role for nitric oxide? Ann NY Acad Sci 893:304–308
Kerschensteiner M, Bareyre FM, Buddeberg BS, Merkler D, Stadelmann C, Bruck W, Misgeld T, Schwab ME (2004) Remodeling of axonal connections contributes to recovery in an animal model of multiple sclerosis. J Exp Med 200:1027–1038
Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M, Lassmann H (2000) Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol 157:267–276
Lassmann H (1998) Pathology of multiple sclerosis. In: Compston A, Ebers G, Lassmann H, McDonald I, Matthews B, Wekerle H (eds) McAlpine’s multiple sclerosis. Churchill Livingstone, London, pp 323–377
Lassmann H, Wekerle H (1998) Experimental models of multiple sclerosis. In: Compston A, Ebers G, Lassmann H, McDonald I, Matthews B, Wekerle H (eds) McAlpine’s multiple sclerosis. Churchill Livingstone, London, pp 409–433
Lassmann H, Brunner C, Bradl M, Linington C (1988) Experimental allergic encephalomyelitis: the balance between encephalitogenic T lymphocytes and demyelinating antibodies determines size and structure of demyelinated lesions. Acta Neuropathol 75:566–576
Linington C, Lassmann H, Morgan BP, Compston DA (1989) Immunohistochemical localisation of terminal complement component C9 in experimental allergic encephalomyelitis. Acta Neuropathol 79:78–85
Linington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y, Lu HC, Lassmann H, Wekerle H (1993) T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol 23:1364–1372
Liu JSH, Zhao ML, Brosnan CF, Lee SC (2001) Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol 158:2057–2066
Medana I, Martinic MA, Wekerle H, Neumann H (2001) Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am J Pathol 159:809–815
Murray PD, McGavern DB, Sathornsumetee S, Rodriguez M (2001) Spontaneous remyelination following extensive demyelination is associated with improved neurological function in a viral model of multiple sclerosis. Brain 124:1403–1416
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343:938–952
Oleszak EL, Zaczynska E, Bhattacharjee M, Butunoi C, Legido A, Katsetos CD (1998) Inducible nitric oxide synthase and nitrotyrosine are found in monocytes/macrophages and/or astrocytes in acute, but not in chronic multiple sclerosis. Clin Diagn Lab Immunol 5:438–445
Pender MP, Sears TA (1985) Vulnerability of the dorsal root ganglion in experimental allergic encephalomyelitis. Clin Exp Neurol 21:211–223
Pender MP, Sears TA (1986) Involvement of the dorsal root ganglion in acute experimental allergic encephalomyelitis in the Lewis rat. A histological and electrophysiological study. J Neurol Sci 72:231–242
Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C (1993) The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol 143:555–564
Redford EJ, Kapoor R, Smith KJ (1997) Nitric oxide donors reversibly block axonal conduction: demyelinated axons are especially susceptible. Brain 120:2149–2157
Reiter CD, Teng RJ, Beckman JS (2000) Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem 275:32460–32466
Smith KJ, Kapoor R, Felts PA (1999) Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 9:69–92
Smith KJ, Kapoor R, Hall SM, Davies M (2001) Electrically active axons degenerate when exposed to nitric oxide. Ann Neurol 49:470–476
Strong MJ, Sopper MM, Crow JP, Strong WL, Beckman JS (1998) Nitration of the low molecular weight neurofilament is equivalent in sporadic amyotrophic lateral sclerosis and control cervical spinal cord. Biochem Biophys Res Commun 248:157–164
Stys PK (1998) Anoxic and ischemic injury of myelinated axons in CNS white matter: from mechanistic concepts to therapeutics. J Cereb Blood Flow Metab 18:2–25
Touil T, Deloire-Grassin MS, Vital C, Petry KG, Brochet B (2001) In vivo damage of CNS myelin and axons induced by peroxynitrite. Neuroreport 12:3637–3644
Trapp BD, Ransohoff R, Rudick R (1999) Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol 12:295–302
Vass K, Heininger K, Schafer B, Linington C, Lassmann H (1992) Interferon-gamma potentiates antibody-mediated demyelination in vivo. Ann Neurol 32:198–206
Viera L, Ye YZ, Estevez AG, Beckman JS (1999) Immunohistochemical methods to detect nitrotyrosine. Methods Enzymol 301:373–381
Waxman SG (2003) Nitric oxide and the axonal death cascade. Ann Neurol 53:150–153
Acknowledgments
This work was supported by the FWF (project P16047-B02) and the European commission (QLG3-CT-2002-00612). The authors wish to thank Ulrike Köck, Angela Kury, and Marianne Leiszer for the excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aboul-Enein, F., Weiser, P., Höftberger, R. et al. Transient Axonal Injury in the Absence of Demyelination: A Correlate of Clinical Disease in Acute Experimental Autoimmune Encephalomyelitis. Acta Neuropathol 111, 539–547 (2006). https://doi.org/10.1007/s00401-006-0047-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0047-y